
News & Events
News & Events
News
June 05, 2017
A new drug target for Blau syndrome
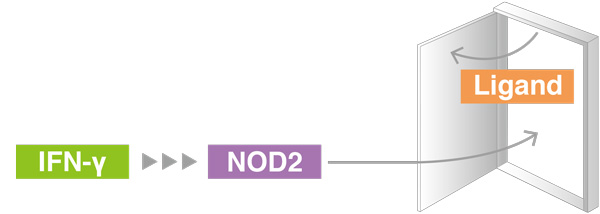
In healthy subjects, immune activation by NOD2 requires both IFN-γ and the ligand of an infection. In Blau syndrome, a mutation allows NOD2 to be activated by IFN-γ alone, causing autoimmune reactions.
Blau syndrome patients leads to excessive inflammation.
Blau syndrome is a rare inflammatory disorder that primarily affects the skin, joints, and eyes, and strikes it victims at preschool age. Pediatrician and CiRA Associate Professor Megumu Saito says there is little in terms of treatment, but a new study by his lab published in the Journal of Allergy and Clinical Immunology reports a new candidate target, IFN-γ.
The cause of Blau syndrome is associated with mutations in the NOD2 gene. The NOD2 protein is a receptor that interacts with ligands found in bacteria, and this interaction initiates an inflammatory response to kill the bacteria. However, in Blau syndrome patients, NOD2 activates the inflammation absent infection.
"Many studies have suggested IFN-γ contributes to Blau syndrome, but its effects on chronic inflammation and NOD2 is unclear," said Saito.
In healthy immune systems, IFN-γ primes macrophages to attack an infection by increasing NOD2 production, increasing the likelihood that a foreign ligand will be bound and thus an inflammatory response. Saito shows, however, that in Blau syndrome patients, NOD2 triggers inflammation following IFN-γ stimulation independent of any ligand.
Using macrophages made from Blau syndrome patient iPS cells, Saito shows that IFN-γ increased NOD2 levels as expected, but that NOD2 went on to automatically produce an inflammatory response regardless if infection had occurred. To validate these findings, his team then forced the NOD2 mutation into iPS cells from healthy donors using CRISPR-Cas9 genome editing technology, finding that macrophages made from these cells showed the same deviant inflammatory properties.
"Our analysis showed that untreated patient macrophages are in a different state from untreated healthy macrophages. Therefore, the inflammation response is different when the macrophages are primed with IFN-γ," explains Saito.
Interestingly, macrophages from patient and healthy donors responded similarly when stimulated with IFN-γ and infectious stimulation, a result that Saito struggles to explain.
"It is paradoxical. One explanation could be that NOD2 uses multiple pathways to activate inflammation. Blau syndrome patients may be biased toward a ligand-independent mechanism," he said.
Saito added the study implies that preventing IFN-γ activation could benefit patients.
"Blocking IFN-γ signaling could be a potential treatment for managing chronic inflammation."
Paper Details
- Journal: Journal of Allergy and Clinical Immunology
- Title: Pluripotent stem cell models of Blau syndrome reveal an IFN-γ-dependent inflammatory response in macrophages
- Authors: Takada S1,2, Kambe N3, Kawasaki Y1, Niwa A1, Honda-Ozaki F1, Kobayashi K1, Osawa M1, Nagahashi A1, Semi K1, Hotta A1,5, Asaka I1, Yamada Y1,5, Nishikomori R4, Heike T4, Matsue H2,6, Nakahata T1, and Saito MK1
- Author Affiliations:
- Center for iPS Cell Research and Application (CiRA), Kyoto University, Kyoto, Japan
- Department of Dermatology, Chiba University Graduate School of Medicine, Chiba, Japan
- Department of Dermatology, Kansai Medical University, Hirakata, Japan
- Department of Pediatrics, Kyoto University Graduate School of Medicine, Kyoto Japan
- Institute for Integrated Cell-Material Sciences, Kyoto University, Kyoto, Japan
- Medical Mycology Research Center, Chiba University, Chiba, Japan






















