RESEARCH
研究紹介
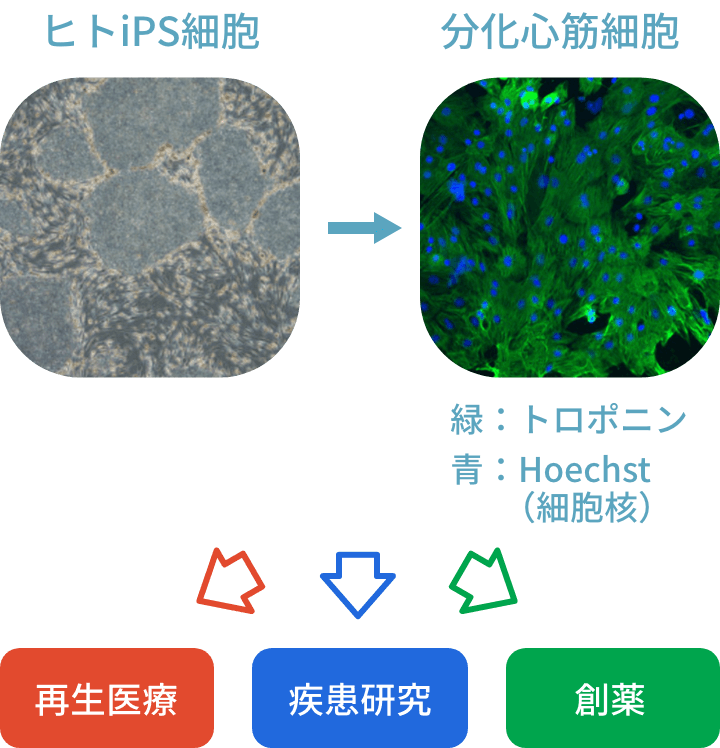
私たちの研究室は心疾患・血液疾患に対してES/iPS細胞を用いて再生医療、創薬、疾患メカニズムの解明を目標として研究を行っています。
現在私たちの研究室では
①再生医療の研究開発
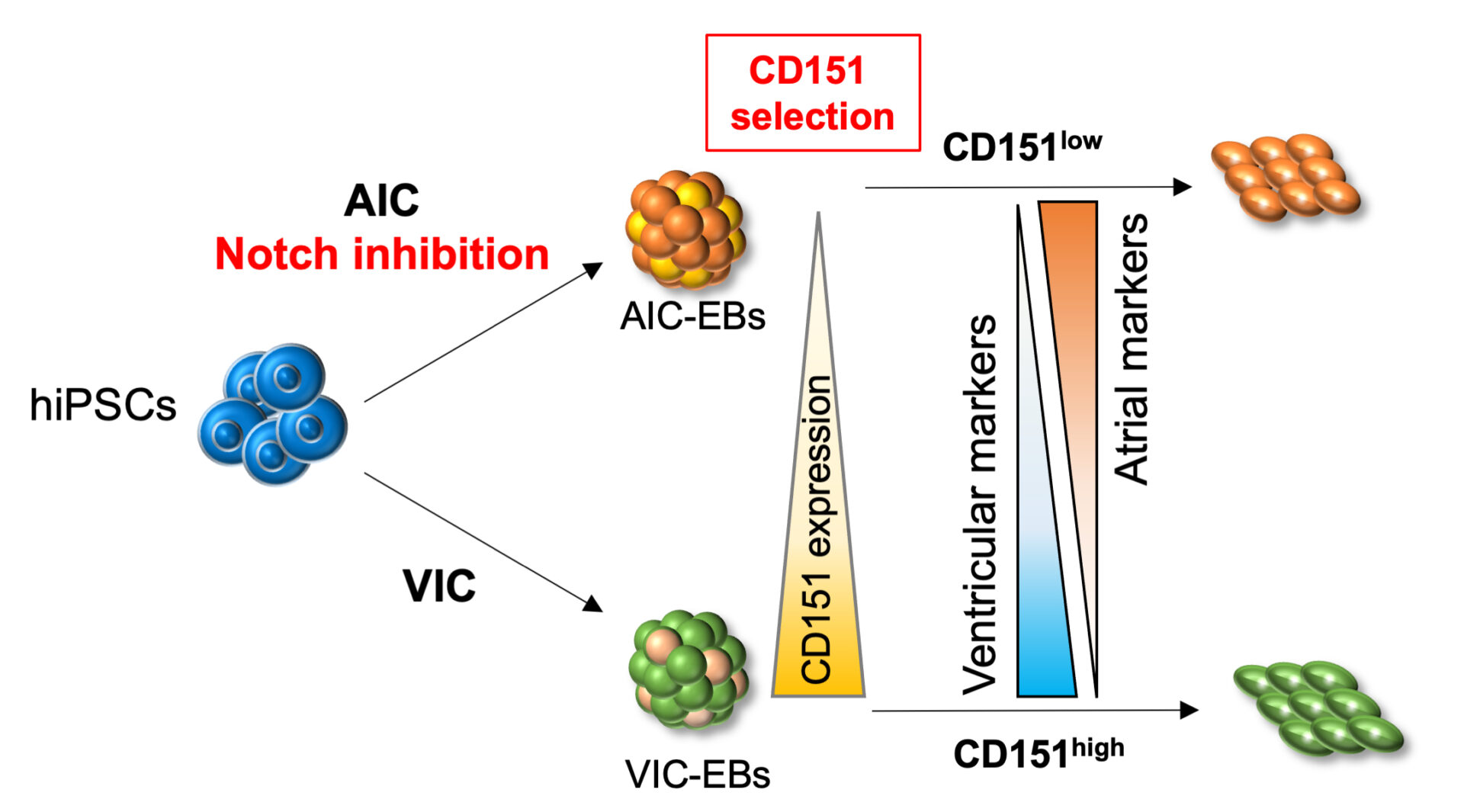
②心疾患、血液疾患の疾患モデル研究・創薬
③分化細胞の成熟制御法の開発
④多能性幹細胞(ES細胞/iPS細胞)の分化能を規定する因子の解明
の4つのテーマに取り組んでいます。
2016年度からはiPS細胞研究所湘南分室においてもT-CiRAプロジェクトの1つとして心筋再生・心不全に対する創薬研究のプロジェクトが始まりました。また2022年からはAltos-CiRA 研究プロジェクトの1つとして心臓の老化・若返り制御に関する研究を開始しています。
私たちの研究室のミッションはiPS細胞研究で見つかったことを臨床応用につなぐことですが、日々の研究において生まれる好奇心を大事にして研究を進めていければと思います。
研究環境
分子生物学実験の基本的な機器から専門的なものまで必要機器を揃えています。研究室はオープンラボにあり,ラボ内外のメンバーとシームレスな交流が盛んにおこなわれています。
MORE

MEMBER