
News & Events
News & Events
News
July 30, 2018
Announcement of physician-initiated clinical trials for Parkinson's disease
Kyoto University Hospital, in partnership with the Center for iPS Cell Research and Application (CiRA), Kyoto University, has planned physician-initiated clinical trials for Parkinson's disease that transplants dopaminergic progenitors1 generated from induced pluripotent stem (iPS) cells. The clinical trial notification was submitted to the Pharmaceutical and Medical Devices Agency (PMDA; the Japanese equivalent of the FDA) on June 4, 2018, and the clinical trials are scheduled to begin on August 1 this year.
Note: The candidate subjects are required to live in Japan, have Japanese public health insurance and can understand the Japanese informed consent form.
1. Names
Selected patients will participate in both of the following clinical trials.
-
a) Kyoto Trial to Evaluate the Safety and Efficacy of iPSC-derived dopaminergic progenitors in the treatment of Parkinson's Disease (Phase I/II)
-
b) Kyoto Trial to Evaluate the Safety and Efficacy of Tacrolimus2 in the iPSC-based Therapy for Parkinson's Disease (Phase III)
2. Objectives
-
a) To evaluate the safety and efficacy of transplanting human iPS cell-derived dopaminergic progenitors into the putamen3 of Parkinson's disease patients.
-
b) To evaluate the safety and efficacy of using tacrolimus for Parkinson's disease patients who received transplantation of human iPS cell-derived dopaminergic progenitors into their putamen.
3. Strategy
Dopaminergic progenitors are generated from iPS cells prepared at the iPS Cell Stock for Regenerative Medicine4 at CiRA and then transplanted into the bilateral putamen of seven subjects (Parkinson's disease patients) at the Kyoto University Hospital.
The source iPS cells were generated from third-party donor blood cells, meaning the transplantations will be allogeneic. Because of a possible transplant rejection, patients will receive a standard immunosuppressant, tacrolimus. Each subject will be observed for two years post transplantation.
4. The cell transplantation surgery
Approximately 5 million iPS cell-derived dopaminergic progenitors will be transplanted by stereotaxic brain surgery5 into the left and right sides of the patient's putamen.
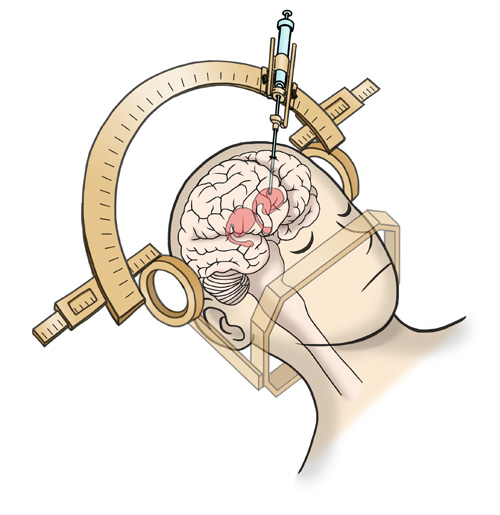
(Image) A 12mm diameter hole will be drilled on the patient's skull,
and cells will be injected using a specialized device.
| April 6, 2018 | Application to the Kyoto University Hospital Institutional Review Board (IRB) |
|---|---|
| April 24, 2018 | Conditional approval from the IRB |
| June 4, 2018 | Submission of the clinical trial notification to the Minister of Health, Labour and Welfare through the PMDA |
| August 1, 2018 | Start of the clinical trials |
1)Dopaminergic progenitors
Dopaminergic neurons produce the neurotransmitter dopamine. In Parkinson's disease, these cells degenerate, resulting in decreased dopamine production. Dopaminergic progenitors differentiate into dopaminergic neurons. Animal studies have shown that transplanted progenitor cells will differentiate into mature dopaminergic neurons, resulting in efficient engraftment in the brain.
2)Tacrolimus
An immunosuppressant commonly used following organ transplantation.
3)Putamen
A region in the basal ganglia that is innervated by midbrain dopaminergic neurons.
4)iPS Cell Stock for Regenerative Medicine
Clinical-grade iPS cells are generated from healthy donors with specific cell types (HLA homozygosity) that are less likely to cause immune rejection in many people, and are stockpiled at CiRA following thorough quality check.
5)Stereotaxic brain surgery
This neurosurgery involves drilling small holes on the patient's skull, through which a needle is entered or electrodes are embedded. One of the current therapies for Parkinson's disease, deep brain stimulation (DBS) surgery, is a type of the stereotaxic brain surgery.
Reference






















