
Research Activities
Research Activities
Publications
February 14, 2014
Method developed for using human iPS cells to create stable and large-scale supply of platelets
A research team led by CiRA Professor Koji Eto has succeeded in generating megakaryocytes with self-replicating ability from human iPS cells, establishing a method for large-scale platelet production. The findings of the research was published on February 13, 2014 (U.S. Eastern time), in the U.S. scientific journal Cell Stem Cell.
Although it had been possible before now to create platelets from iPS cells, producing platelets on the scale required for transfusion had been impractical. By focusing attention on the megakaryocyte cells from which platelets originate, Eto 's group including researcher Sou Nakamura, the first author of the paper, have made it feasible to produce platelets on an increased scale large enough for use in practical medical treatment.
Platelets, blood cells which play an important role in hemostasis, arise through fragmentation from cells known as megakaryocytes. As they circulate in the blood, they are either used in hemostasis or destroyed after a fixed lifespan. Since they are not capable of autonomous cell division, constant production from megakaryocytes is required to maintain the platelet population.
Currently, patients with blood diseases that cause serious anemia or hemorrhage are obliged to rely on transfusion of blood products made with donor blood. However, for demographic and other reasons, the number of blood donors is on the decline in Japan. According to statistics published by the Ministry of Health, Labour and Welfare, the shortage of blood donors means that Japan will experience a shortfall of 20% in its required supply of transfusion products by 2027.
Moreover, platelets need to be stored at room temperature in order to maintain their functions and are only usable for four days after blood collection. This makes it difficult to supply platelets in the amount required at the time required. To remedy this situation, a system is needed that can produce platelets and other blood products without having to rely on donors.
In 2010, Eto's team announced the successful production of platelets from dermal cell-derived iPS cells through in vitro culture. However, a single transfusion to one patient requires 200 to 300 billion platelets, whereas methods in use so far have only been able to produce around one billion units. This time, the team focused its attention on megakaryocytes, the progenitor cells of platelets, and attempted to induce megakaryocytes with long-term self-replicating ability.
In the methods used so far to generate platelets from iPS cells, around seven billion human iPS cells are needed to produce the 100 billion platelets required for a transfusion, and it takes around 26 days before the platelets can finally be harvested. By contrast, the method developed by the researchers this time allows platelets to be harvested in five days with the use of 25 billion self-replicating megakaryocyte progenitor cells. The equipment used in the culture process is also simple, with the switch from laboratory-type culture dishes (10 mL) to bags (1-500 L) enabling large-scale culture without the need for complex equipment.
This system offers the possibility of using iPS cells with the HLA phenotypes, which are common among the Japanese population, to create a stock of megakaryocytes for preparation of platelet products, thereby ensuring a stable supply of platelet products to patients with the HLA or special platelet (HPA) phenotypes, for which donors are hard to find.
Our research team developed its megakaryocyte cell production method with a view to future clinical research and clinical trials. The present research also compared a number of methods of immortalizing megakaryocyte cells to identify the most stable production method for use in clinical research or trials. After a clinical trial in a few years , the ultimate aim is to achieve practical application in 10 years' time.
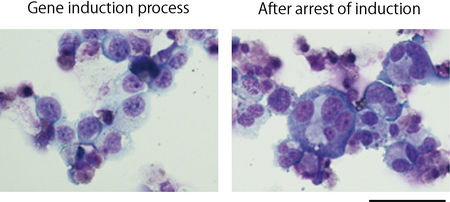
Fig. Megakaryocyte cells derived from a cell line immortalized after polyploidization and maturation
Immature megakaryocytes displaying expression of three genes (left) and megakaryocytes matured after arrest of induced gene expression (right). By controlling the gene expression, megakaryocytes start producing platelets.
Giemsa staining. Scale bar = 50µm.
Paper Details
- Journal: Cell Stem Cell
- Title: Expandable megakaryocyte cell lines enable clinically-applicable generation of platelets from human induced pluripotent stem cells
- Authors: Toshikazu Araoka, Shin-ichi Mae, Yuko Kurose, Motonari Uesugi, Akira Ohta,
Shinya Yamanaka, and Kenji Osafune






















