
Research Activities
Research Activities
Publications
August 18, 2014
Gene introduction via piggyBac vector improves blood coagulation function in a mouse model of hemophilia A
A CiRA research team led by Assistant Professor Akitsu Hotta has succeeded in improving blood coagulation function in a mouse model of hemophilia A by introducing the full-length blood coagulation factor gene with the help of a piggyBac vector, which is a type of transposon. The results of the research, which were carried out in a joint study with the research team of Lecturer Hideto Matsui at Nara Medical University, were published on August 15, 2014 (US Eastern time), in the US scientific journal PLOS ONE.
Hemophilia is a congenital disorder in which the proteins that cause blood to clot is deficient or absent, with the result that bleeding is difficult to stop. The form of the disease known as hemophilia A is a hereditary disorder caused by the absence of procoagulant factor VIII (FVIII) due to the mutated FVIII gene on the X chromosome. The main form of therapy is administration of preparations of the deficient Factor VIII protein, but as this becomes inactive within a short period, patients with severe forms of the disease need repeated administrations every few days. If a form of gene therapy could be developed to induce stable long-term expression of the FVIII gene that secretes Factor VIII, an improvement in patient quality of life could be achieved.
In the field of gene therapy, when introducing normal genes from outside to replace mutated genes, viral vectors based on a retrovirus or similar carrier are the most frequently used method because of their higher rate of success. However, this method carries the constant risk of immune response to the inserted virus-derived protein or unexpected mutations in the viral sequence. A further issue is that, as the FVIII gene is very large, it exceeds the size that can be delivered by a virus. Up till now, the form of the gene normally used has therefore been one with the central section removed, known as the B domain-deleted FVIII gene.
To resolve these issues, the research team turned its attention to a transposon vector known as a piggyBac vector as a vehicle for introducing the FVIII gene. The present study investigated whether use of this piggyBac vector would allow the full-length FVIII gene to be inserted without using a viral vector.
First, a variety of genes of different sizes were incorporated into the piggyBac vector and then introduced into human kidney-derived cultured cells. The smaller the gene, the higher the success rate of delivery. With the human full-length FVIII gene, although the delivery success rate was low, it was found to be about the same as with the B domain-deleted FVIII gene.
Next, the B domain-deleted FVIII gene and the full-length FVIII gene were each introduced into human kidney-derived cultured cells and human iPS cells, following which measurement was made of the amount of gene expression and the activity of the secreted FVIII protein. It was found that, compared to the B domain-deleted form, the full-length FVIII factor produced higher gene expression in both types of cell when introduced, and showed higher FVIII protein activity in the kidney-derived cultured cells. However, in the human iPS cells, FVIII protein activity was lower than the kidney-derived cultured cells, suggesting that the protein secretion pathway was immature. Among the tasks researchers will need to work on going forward is finding a way to boost the secretion of FVIII protein by inducing iPS cells to differentiate into the appropriate cell type.
The next step was to introduce a piggyBac vector carrying the full-length FVIII gene into the mouse hemophilia A model using hydrodynamic injection. This was confirmed to result in secretion into the blood of FVIII protein that retained activity for more than 300 days. It was also confirmed that the volume of FVIII protein secreted could be further increased by performing gene delivery via injection of the piggyBac transposon vector three times at four-week intervals. Lastly, on measuring tail bleeding time in the mouse model of hemophilia A, it was found that, without vector injection, bleeding continued for an average of 18 minutes, whereas in mice that had undergone gene introduction via injection of the piggyBac vector, bleeding was arrested in an average of 6 minutes, demonstrating a recovery in blood coagulation function.
The present research indicates that the use of a piggyBac vector makes it possible to deliver the full-length FVIII gene and induce stable gene expression. This method is expected to make future contributions to gene therapy for hemophilia and other diseases.
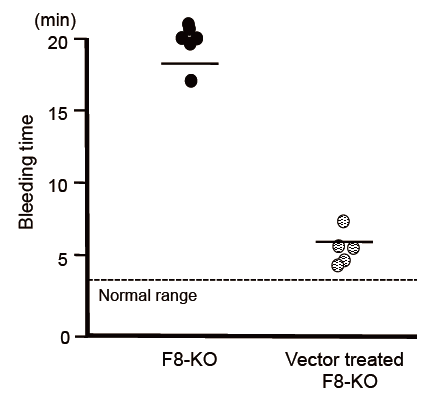
Fig. The full-length FIII gene introduction via piggyBac vector improved blood coagulation function
Bleeding time in the hemophilia A mouse model was compared without and with vector injection. In mice that had undergone gene introduction, bleeding time was shortened, demonstrating a recovery in blood coagulation function.
F8-KO: Mice without vector injection
Vector treated F8-KO: Mice treated by gene introduction via vector injection
Paper Details
- Journal: PLOS ONE
- Title: Delivery of Full-Length Factor VIII Using a piggyBac Transposon Vector to Correct a Mouse Model of Hemophilia A
- Authors: Hideto Matsui, Naoko Fujimoto, Noriko Sasakawa, Yasuhide Ohinata, Midori Shima, Shinya Yamanaka, Mitsuhiko Sugimoto, and Akitsu Hotta






















