
Research Activities
Research Activities
Publications
August 29, 2018
Making new cartilage from reprogrammed cells
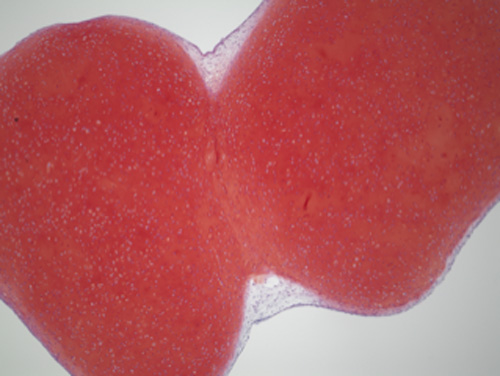
Photo of two iPS-Cart adhering at the cartilage component (red).
Cartilage is the tissue that gives joints their mobility. It is extraordinarily robust and built to last a lifetime. Ironically, while durable, unlike other tissue in the body, cartilage is incapable of repairing on its own. CiRA Professor and Orthopedic Surgeon Noriyuki Tsumaki is using iPS cell technology to produce a surgical option that repairs cartilage defects. In the newest study from his lab, he reports how his iPS-Cart adhere to one another to form new cartilage.
"Our iPS-Cart are small particles 1-3 mm in diameter. We plan to transplant about 100 iPS-Cart depending on the size of the cartilage defect," he says.
Once transplanted, the iPS-Cart integrate with the surrounding cartilage of the patient and also with each other to form one continuous cartilage that Tsumaki expects will function as well if not better than prior to the defect.
"The best surgical option for cartilage defects is transplanting cartilage tissue. Our iPS-Cart show good integration into pig and other animals. We do not know how they integrate with one another," says Dr. Xike Chen, a Ph.D. student in the Tsumaki lab.
iPS-Cart can be divided into an interior that constitutes cartilage and an exterior that constitutes what Chen calls a "perichondrium-like membranous tissue."
"The perichondrium surrounds the cartilage of developing bone. Perichondrium also functions in the growth and repair of cartilage," she says.
She found that two iPS-Cart would first adhere at their perichondrium-like membranous tissue, but after less than two months the adhesion would reach the interior cartilage, purging the perichondrium-like membranous tissue to leave a structure that resembles one large iPS-Cart.
Chen then looked for biological factors naturally found in cartilage that could promote the adhesion.
"We found FGF18 was expressed more in the perichondrium-like membranous tissue than cartilage of iPS-Cart. We added FGF18 to see if it accelerates the integration," she says.
Indeed, adding FGF18 shortened the adhesion time of the perichondrium-like membranous tissue in iPS-Cart, suggesting that combining FGF18 treatment with an iPS-Cart transplant could quicken the recovery.
Tsumaki explains that understanding how iPS-Cart adhere and what conditions are best for the adhesion is a key step to using iPS cells for cartilage therapies.
"My goal is to provide good treatment options to patients with cartilage damage. We are developing iPS-Cart to be used in patients," he says.
Paper Details
- Journal: Tissue Engineering Part A
- Title: Integration Capacity of Human Induced Pluripotent Stem Cell-Derived Cartilage
- Authors: Xike Chen1, Akihiro Yamashita1, Miho Morioka1, Toshika Senba1, Takashi Kamatani1, Akira Watanabe1, Azuma Kosai1, and Noriyuki Tsumaki1
- Author Affiliations:
- Center for iPS Cell Research and Application (CiRA), Kyoto University, Kyoto, Japan






















