
News & Events
News & Events
News
August 09, 2016
Transplantation of non-cardiomyocytes gives best recovery for heart dysfunction
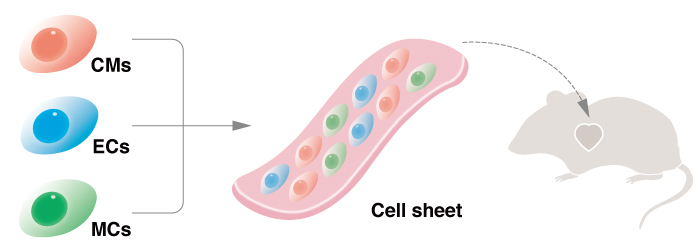
Cardiac tissue comprised of cardiomyocytes, endothelial cells and vascular mural cells resulted in the best therapeutic effect in rats with destroyed cardiomyocytes.
Regenerative medicine for heart failure has primarily depended on replacing the lost cardiomyocytes. However, this alone rarely achieves sufficient recovery, even though cardiomyocytes are responsible for heart function. "A mix of cardiomyocytes and non-cardiomyocytes gives the best result," says CiRA Professor Jun Yamashita. As experts on iPS cell differentiation to cardiac cells, the Yamashita team partnered with pediatric cardiologist Bradley B. Keller and his group at the University of Louisville, Kentucky, who are experts on the engineering of cardiac tissues. The collaboration has recently led to a new report in Scientific Reports that shows how transplants of human iPS cells differentiated into three cell types, cardiomyocytes, endothelial cells (ECs) and vascular mural cells (MCs), cause good recovery in rats suffering from myocardial infarctions. "These are important preliminary experiments for clinical work," says Hidetoshi Masumoto, a cardiovascular surgeon and specially-appointed assistant professor at CiRA who has spent the last two years in the Keller lab.
iPS cells can make a large number of cardiomyocytes compared to other sources, but with a drawback; the resulting cardiomyocytes are generally immature compared with cells in the host heart, leading to suboptimal recovery. The study shows that by adding ECs and MCs into the transplanted tissue, cardiac function is significantly improved. This improvement was attributed to structural gains in the transplant, including better electromechanical capacity, stiffness and geometry. Successful heart function requires a synchronicity in all cells constituting the heart. To achieve this synchronicity, the cells must be aligned properly so that when one cell is activated, neighbouring cells are subsequently activated or deactivated appropriately. Transplants that included ECs and MCs showed better alignment in the heart, resulting in clearly visible sarcomeres and better structural maturation. This maturation facilitated blood flow from the host heart to the transplant. Masumoto surmised that this effect is due to genetic changes. "We found that MCs promoted the expression of genes responsible for maturation," he said. Further study of these genes is expected to reveal key molecular pathways for maximal heart recovery.
The two groups are now considering larger animals with hearts whose function more closely resemble human heart than rats. "We want to determine the right mix of cells for best therapeutic outcomes," said Yamashita.
Paper Details
- Journal: Scientific Reports
- Title: The myocardial regenerative potential of three-dimensional engineered cardiac tissues composed of multiple human iPS cell-derived cardiovascular cell lineages
- Authors: Hidetoshi Masumoto1,2,3,*, Takeichiro Nakane1,2,3,*, Joseph P Tinney1,4, Fangping Yuan1,4, Fei Ye1,4, William J Kowalski1,4, Kenji Minakata3, Ryuzo Sakata3, Jun K Yamashita2, Bradley B Keller1,4,a
- Author Affiliations:
- Kosair Charities Pediatric Heart Research Program, Cardiovascular Innovation Institute, University of Louisville, Louisville, Kentucky, The United States of America
- Department of Cell Growth and Differentiation, Center for iPS Cell Research and Application (CiRA), Kyoto University, Kyoto, Japan
- Department of Cardiovascular Surgery, Kyoto University Graduate School of Medicine, Kyoto, Japan
- Department of Pediatrics, University of Louisville School of Medicine, Louisville, Kentucky, The United States of America






















