
News & Events
News & Events
News
May 25, 2018
CiRA scientists create artificial cells to battle cancer
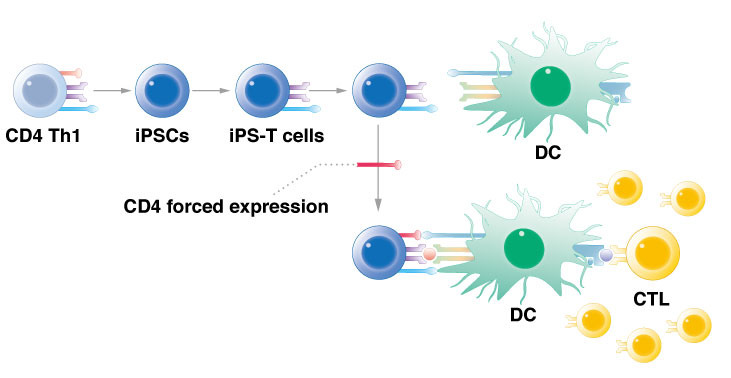
CiRA researchers show that reprogramming T cells and then forcing the expression of the CD4 gene activates immune cells to attack cancer.
Researchers at CiRA have used iPS cell technology to create an artificial type of immune cell that acts against cancer cells. The artificial cells have properties of innate lymphoid cells, a type of immune cell that reacts quickly to infection or injury. By activating a single gene, CD4, the innate lymphoid cells can be converted into what the researchers call "T helper-like cells." The new study, which can be read in Stem Cell Reports, shows the potential of these cells in cancer immunotherapy against leukemia.
The immune system is designed to detect and kill cancer cells by recognizing signature peptides on the cancer cell surface. However, cancer has evolved to evade immune cells. In response, cancer researchers are looking for ways that strengthen the detection capability.
According to CiRA Associate Professor Shin Kaneko, among the many types of immune cells, "CD4 T helper cells are effective at preventing the escape of immune surveillance by inducing a polyclonal immune response called antigen spreading." Antigen spreading expands the types of peptides on the cancer cell to which the immune cells recognize.
Once they recognize the cancer peptide, CD4 T helper cells will activate cytotoxic T lymphocytes, the cells that are ultimately responsible for killing the cancer cells. "CD4 T helper cells activate dendritic cells to induce cytotoxic T lymphocytes that act on the cancer peptide," continued Kaneko.
Cancer cells thrive when the immune system functions sub-optimally. In previous works, Kaneko's research team demonstrated that by using iPS cell reprogramming technology, they could "rejuvenate" cytotoxic T lymphocytes so that they more effectively kill the cancer. In the present study, he expanded this strategy to work on cells responsible for cancer detection, namely, T helper cells.
The rejuvenation of immune cells involves reprogramming them into iPS cells and then turning them back to their original form. In the new study, his team rejuvenated CD4 T helper cells that respond to a leukemia peptide, b3a2. However, unlike his previous work with cytotoxic T lymphocytes, differentiation of the iPS cells unexpectedly produced cells more akin to innate lymphoid cells, not rejuvenated CD4 T helper cells.
"That wasn't our goal. Innate lymphoid cells are a new type of cell. They don't have antigen specificity and have very general helper function," said Kaneko. In addition, they have a short lifespan and are localized, whereas CD4 helper T cells have a long lifespan and operate throughout the body.
However, his team showed that manipulating these innate lymphoid cells could convert them to take CD4 T helper cell activity.
"If we force CD4 gene expression and keep the original TCR expression, we can get enough signal for helper activity in an antigen-specific manner," Kaneko added. Keeping the original TCR expression is crucial for the immune cells to respond to the cancer antigen and not other cells in the body.
To test the effectiveness of their "CD4 T helper-like cells," the scientists used a leukemia mouse model. The rejuvenated innate lymphoid cells with forced CD4 expression were used to prime cytotoxic T lymphocytes. Injection of the primed cytotoxic T lymphocytes into the mice significantly inhibited tumour growth.
While Kaneko calls his cells "very artificial," he believes they are just another example of how iPS cell reprogramming technology could be used to fight cancer.
"It is a way to give antigen specificity to innate lymphoid cells," he said.
Paper Details
- Journal: Stem Cell Reports
- Title: Generation of TCR-expressing innate lymphoid-like helper cells that induce cytotoxic T cell-mediated anti-leukemic cell response
- Authors: Norihiro Ueda1,2,4, Yasushi Uemura3,4, Rong Zhang3,4, Shuichi Kitayama1, Shoichi Iriguchi1, Yohei Kawai1, Yutaka Yasui1, Minako Tatsumi4, Tatsuki Ueda1, Tian-Yi Liu4,5, Yasutaka Mizoro1, Chihiro Okada1, Akira Watanabe1, Mahito Nakanishi6, Satoru Senju7, Yasuharu Nishimura7, Kiyotaka Kuzushima4, Hitoshi Kiyoi2, Tomoki Naoe8, and Shin Kaneko1
- Author Affiliations:
- Center for iPS Cell Research and Application (CiRA), Kyoto University, 53 Shogoin, Kawahara-cho, Sakyo-ku, Kyoto 606-8501, Japan
- Department of Hematology and Oncology, Nagoya University Graduate School of Medicine, 65 Tsurumai-cho, Showa-ku, Nagoya 466-8550, Japan
- Division of Cancer Immunotherapy, Exploratory Oncology Research & Clinical Trial Center, National Cancer Center (NCC), 6-5-1 Kashiwanoha, Kashiwa, Chiba 277-8577, Japan
- Division of Immunology, Aichi Cancer Center Research Institute (ACCRI), 1-1 Kanokoden, Chikusa-ku, Nagoya, 464-8681, Japan
- Key Laboratory of Cancer Center, Chinese PLA General Hospital, 28 Fuxing Road, Beijing 100853, China
- Research Center for Stem Cell Engineering, National Institute of Advanced Industrial Science and Technology (AIST), 1-1-1 Higashi, Tsukuba, Ibaraki 305-8561, Japan
- Department of Immunogenetics, Graduate School of Medical Sciences, Kumamoto University, 1-1-1 Honjo, Chuo-ku, Kumamoto 860-8556, Japan
- National Hospital Organization Nagoya Medical Center, 4-1-1, Sannomaru, Naka-ku Nagoya, 460-0001, Japan






















