
News & Events
News & Events
News
January 24, 2020
Allogeneic iPS cell-based therapy for articular cartilage injury
Kyoto University Hospital and the Center for iPS Cell Research and Application (CiRA) have been conducting research together on the use of iPS cells to repair damaged knee joint cartilage. On November 7, 2019, they submitted an application to the Japan Ministry of Health, Labour and Welfare (MHLW) to begin clinical experiments. The MHLW confirmed the application met the criteria for regenerative medicine, as outlined below.
Articular cartilage prevents the two bones in a joint from coming in direct contact, allowing for smooth and painless joint movement.
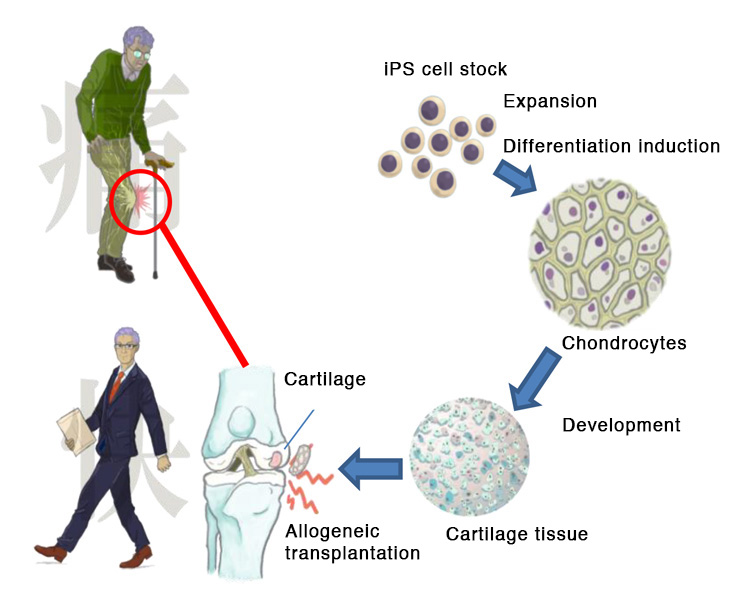
The regenerative medicine in this proposal is intended to benefit patients who have damaged the articular cartilage in the knee joint. These patients may feel pain or discomfort in the knee when undergoing simple or frequent movement like walking. Current treatment usually involves the transplantation of healthy cartilage to the damaged site.
In the proposed research, a sufficient amount of healthy cartilage will be prepared from iPS cells and used for the transplant. Animal experiments have confirmed that this iPS cell-derived cartilage does not produce tumors. Further, the cartilage regenerated at the location of the transplantation. The proposed research seeks to confirm the same effects when the cartilage is transplanted into patients with articular cartilage damage in the knees.
| October 15, 2019 | Approval given by a special committee at Kyoto University |
|---|---|
| November 7, 2019 | Proposal submitted to the MHLW |
| December 11, 2019 | First examination by MHLW review board on regenerative medicine |
| January 24, 2020 | Second examination by MHLW review board on regenerative medicine |
- Clinical trial name:Allogeneic iPS cell-based therapy for articular cartilage therapy
- Purpose:To test the safety of iPS cell-derived cartilage transplanted into patients with articular cartilage damage to the knee joint.
- Study design:Single center, open-label, uncontrolled study
- Target disease:Knee joint cartilage damage
- Primary endpoint:Frequency of adverse events and of tumors
- Number of patients:4
- Observation period:One year following the transplantation
- Patient recruitment:Because the purpose of the study is to test safety and the small number of patients, there will be no open recruitment.
- Allogeneic iPS cell-derived cartilage:iPS cells from the iPS Cell Stock at CiRA are used to produce chondrocytes, which are further processed to produce the cartilage used for the transplantation.
-
Principle Investigator:
Prof. Noriyuki Tsumaki, CiRA Dept. of Clinical Application -
Principle Clinician:
Prof. Shuichi Matsuda, Kyoto University Hospital, Dept. of Orthopedics -
Institutions:
-
Center for iPS Cell Research and Application (CiRA), Kyoto University
Production and quality control of the iPS cell-derived cartilage -
Institute for Advancement of Clinical and Translational Science (iACT), Kyoto University
Preparation and support for the clinical research
-
Center for iPS Cell Research and Application (CiRA), Kyoto University
This clinical study is supported by the following organizations.
-
Japan Agency for Medical Research and Development (AMED)
-
Research Center Network for Realization of Regenerative Medicine (B)
「iPS cell-derived cartilage therapy for cartilage disease」 -
Regenerative Medicine Research Project
「allogeneic iPS cell-derived cartilage transplantation for regenerative medicine」
-
Research Center Network for Realization of Regenerative Medicine (B)
- Asahi Kasei






















