
Research Activities
Research Activities
Publications
January 06, 2022
Synthetic gene circuits that improve stem cell quality
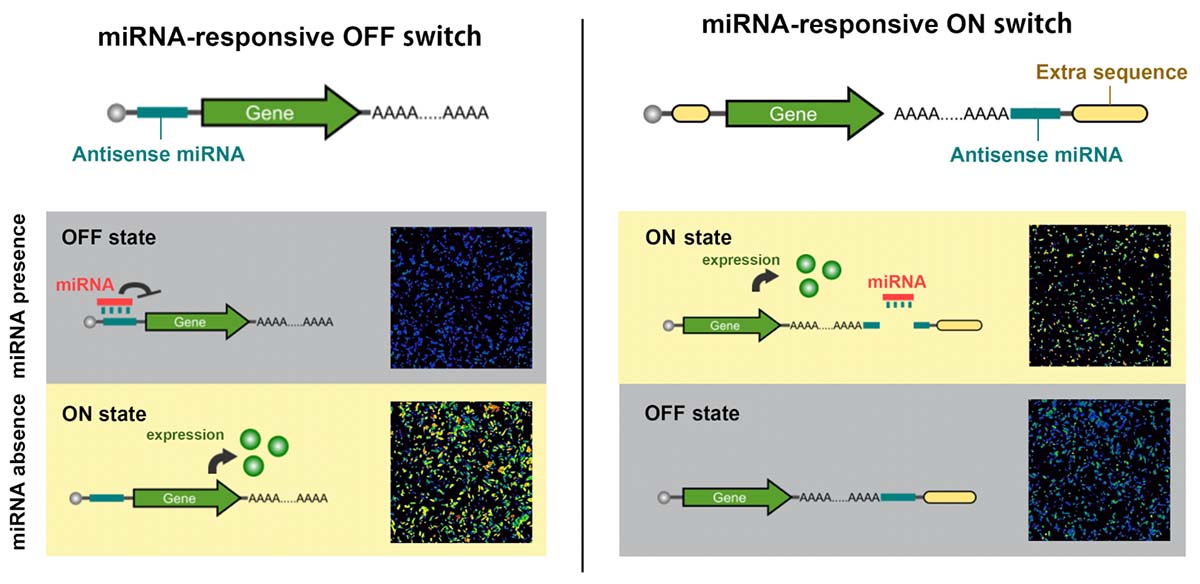
Schematic illustration of miRNA-OFF and miRNA-ON switches
Because iPS cells can be made into just about any cell type in the body, they have great promise for cell therapies. One major problem, however, is that not all reprogramming cells successfully become iPS cells, resulting in an unwanted cell mixture. Further, when differentiating iPS cells, some cells are only partially differentiated, again leaving an unwanted cell mixture. To extract the desired cell types, CiRA researchers report in Science Advances new synthetic RNA technology consisting of ON and OFF switches. These switches control specific genes to kill contaminating cells while leaving desired cells unscathed at rates superior to standard techniques.
"The standard method for cell purification is to use fluorescence-activated cell sorting or magnetic-activated cell sorting," said CiRA Assistant Professor Yoshihiko Fujita, one of the authors of the study. "But antibodies are needed, which makes these approaches costly, and the process damages many cells."
The Hirohide Saito lab, in which Fujita is a member, has therefore been developing synthetic RNA they call "RNA switches" as an alternative. Unlike antibodies, which react with proteins on the surface of the cell, the RNA switch binds with miRNA naturally expressed inside the cell.
The primary function of miRNA is to prevent the translation of RNA into proteins. Thus, the RNA switch, when transfected into a cell, will translate proteins so long as it is unbound to miRNA.
There are several thousand types of miRNA in a cell, but the RNA switch includes a complementary sequence to specific miRNA. Therefore, by designing the RNA switch to translate a protein that causes cell death, only cells that highly express the miRNA that binds to the complementary sequence will survive.
In this system, all RNA switches operate as OFF switches, in that the presence of high miRNA turns the switch OFF. These switches can be used to purify cells, but run into problems if done at large scale, Saito explained.
"There are two problems. First, the amount of RNA switch transfected will influence the purity. Second, there is leakiness, which means some unwanted cells will survive or some wanted cells will perish," he said.
As a solution, his research team prepared RNA switches that turn ON when bound by miRNA. To do so required some experimentation on the synthetic RNA structure that constitutes the RNA switch.
In human cells, translated RNA include a poly(A) tail modification. The miRNA ON switch differs from the OFF switch by placing the complementary sequence after this tail rather than before it.
"We added the complementary sequence and an extra sequence after the poly(A) tail. The extra sequence prevents the RNA from being translated. When the miRNA binds to the sequence, it is expected to cleave the sequence after the poly(A) tail, allowing the protein to be translated," explained Fujita.
Transfecting cells with both miRNA ON and OFF switches reduced concerns about leakiness and amount.
"Generally, the transfection efficiency depends on the cell type, number of cells and so on. By pairing ON and OFF switches, we do not have to worry as much about strictly controlling the amount of RNA for the transfection," said Saito.
Saito and his colleagues tested this strategy by designing the RNA ON switch to translate Barnase, a ribonuclease that degrades RNA and also kills cells, and the RNA OFF switch to translate Barstar, an inhibitor of Barnase, to eliminate any leaky Barnase expression.
In addition, the RNA switches were designed to bind to miR-302a-5p, a miRNA highly expressed in iPS cells, or miR-208a-3p or miR-1-3p, two miRNAs highly expressed in cardiomyocytes. Upon transfecting the cells with these switches, the researchers could purify iPS cells at more than 99% and cardiomyocyte at 95%, numbers that surpass purities levels achieved with standard methods.
Moreover, because RNA molecules are relatively safe and in principle RNA switches can be designed to interact with any type of miRNA, Saito is optimistic about the promise of this technology for clinical research.
"Our method is applicable to a wide range of cell types that can then be used to study disease, drugs, and cell therapies," he said.
Paper Details
- Journal: Science Advances
- Title: A versatile and robust cell purification system with an RNA-only circuit composed of microRNA-responsive ON and OFF switches
- Authors: Yoshihiko Fujita1, Moe Hirosawa1, Karin Hayashi1, Takehsi Hatani2, Takuya Yamamoto1,3,4,
and Hirohide Saito1 - Author Affiliations:
- Department of Life Science Frontiers, Center for iPS Cell Research and Application (CiRA), Kyoto University, Kyoto, Japan
- Department of Cell Growth and Differentiation, Center for iPS Cell Research and Application (CiRA), Kyoto University, Kyoto, Japan
- Institute for the Advanced Study of Human Biology (WPI-ASHBi), Kyoto University, Kyoto, Japan
- Medical-risk Avoidance based on iPS Cells Team, RIKEN Center for Advanced Intelligence Project (AIP), Kyoto, Japan






















