
News & Events
News & Events
News
August 27, 2018
Reprogrammed patient cells explain how the environment triggers muscular dystrophy
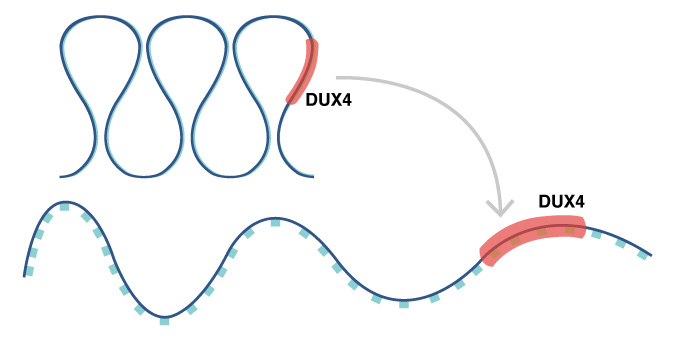
In FSHD, a change in the chromatin structure results in abnormal expression of the DUX4 gene.
Muscular dystrophy describes a set of diseases of muscle weakness and eventually muscle death. In most cases, all muscles are affected, but facioscapulohumeral muscular dystrophy (FSHD) is unusual in that muscles primarily in the face (facio-), shoulder blades (scapulo-) and upper arms (humeral) are victim. FSHD comes in two forms, FSHD1 and FSHD2, but both involve mutations associate with chromosome 4. These mutations open the chromosome to express the gene DUX4 at abnormally high levels. However, a new study by CiRA researchers suggests that patients with these mutations are at higher risk when their cells are exposed to oxidative stress.
"If we can close the chromatin structure, we could stop the expression," hypothesizes CiRA Ph.D. student Mitsuru Sasaki-Honda, who was the first author on the study.
Yet despite the mutation being necessary for the disease, because patients show a variety of clinical symptoms, some scientists believe that something outside the cell could also be a cause.
"The varying clinical characteristics of the disease strongly support the existence of exogenous factors that modulate DUX4 expression," says CiRA Associate Professor Hidetoshi Sakurai, who has based his career on finding cures for muscular dystrophy diseases.
To find these exogenous factors, Sasaki-Honda prepared muscle cells from the iPS cells of FSHD1 or FSHD2 patients. Although these muscle cells naturally expressed higher levels of DUX4 than did muscle cells from iPS cells of healthy donors, the differences were significantly amplified when exposing the cells to hydrogen peroxide, a molecule that simulates oxidative stress on the cell.
"Oxidative stress leads to an imbalance in free radicals in the cell. It is associated with many diseases," says Sasaki-Honda.
Correcting the mutation using CRISPR-Cas9 gene editing technology attenuated the hydrogen peroxide effect by modifying the chromatin structure in a way that prevented the DUX4 expression.
"The chromatin is in a relaxed state in patients. In healthy people, it is in a heterochromatic state," says Sakurai.
Further study showed that oxidative stress increased DUX4 expression by activating the molecule ataxia-telangiectasia mutated kinase.
However, ataxia-telangiectasia mutated kinase is involved in DNA repair, which suggests modifying its behaviour is unwise. Until a cure is found, ways to considering slowing down the disease by reducing oxidative stress through lifestyle changes like diet and exercise should be considered.
Paper Details
- Journal: Human Molecular Genetics
- Title: A Patient-derived iPSC Model Revealed Oxidative Stress Increases Facioscapulohumeral Muscular Dystrophy-causative DUX4
- Authors: Mitsuru Sasaki-Honda1,2, Tatsuya Jonouchi1, Meni Arai1,3, Akitsu Hotta1, Satomi Mitsuhashi4,5, Ichizo Nishino5, Ryoichi Matsuda2,6, Hidetoshi Sakurai1
- Author Affiliations:
- Center for iPS Cell Research and Application (CiRA), Kyoto University, Kyoto, Japan.
- Department of Biological Sciences, Graduate School of Science, the University of Tokyo, Tokyo, Japan.
- Agricultural and Environmental Engineering, Faculty of Agriculture, Kyoto University, Kyoto, Japan.
- Department of Human Genetics, Yokohama City University Graduate School of Medicine, Yokohama, Japan.
- Department of Neuromuscular Research, National Institute of Neuroscience, National Center of Neurology and Psychiatry, Tokyo, Japan.
- Department of Life Sciences, Graduate School of Arts and Sciences, the University of Tokyo, Tokyo, Japan.






















