Research Activities
Research Activities
Principal Investigators
Dept. of Clinical Application
Hidetoshi Sakurai (Associate Professor)

Hidetoshi Sakurai M.D., Ph.D.
- Lab Website
- Research Progress in FY2024
- Contact: sakurai-g*cira.kyoto-u.ac.jp
- Please change * to @
Research Overview
Our laboratory aims to establish remedies for refractory muscle diseases, specifically muscular dystrophy. With iPS cells, we now have two ways to attain such treatments: cell transplantation and drug development. For cell transplantation, we use progenitor cells differentiated from iPS cells as a cell source and evaluate therapeutic effects in disease model animals. For drug development, we employ iPS cells established from somatic cells taken from patients to reproduce disease states in vitro as a tool for drug discovery.
Development of cell transplantation
As cell transplant therapy for muscular dystrophy, we envisage regenerating and repairing damaged muscle fibers by transplanting skeletal muscle stem cells generated from our iPS Cell Stock for Regenerative Medicine. The aim is to achieve successful engraftment following in vivo differentiation into satellite cells, a type of skeletal muscle stem cell. The in vivo proliferation of these satellite cells will, in turn, contribute to repeated regeneration and exert further therapeutic effects by increasing the amount of healthy muscle fibers. To treat a muscular disease caused by defective interstitial proteins, we aim to transplant iPS cell-derived mesenchymal stromal cells generated from the iPS Cell Stock and leverage them as in vivo factories to produce and deposit the protein of interest into the interstitial tissue.
Construction of disease models
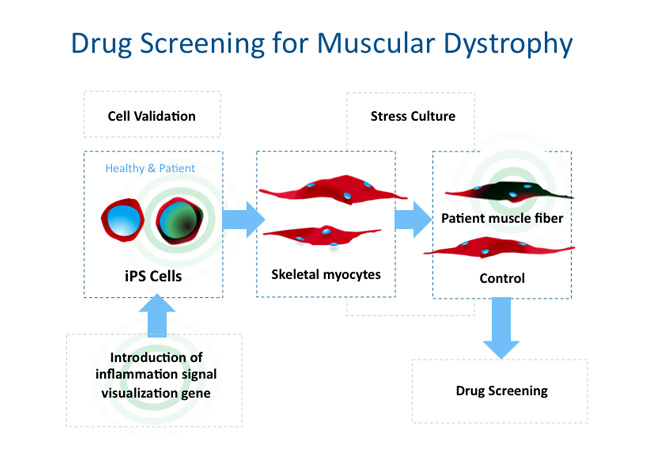
iPS cell technology provides the advantage of creating pluripotent stem cells from anyone. Leveraging this powerful technology, we are working with pediatricians and neurologists to construct disease models using iPS cells derived from muscular dystrophy patients. Concurrently, we are testing methods for highly efficient differentiation of adult skeletal muscles and investigating the possibility of reproducing the disease state of muscular dystrophy by culturing differentiation-induced skeletal muscle in systems incorporating physical stress.
Meanwhile, through joint research with pharmaceutical companies mainly, we have developed a screening system based on disease models we successfully established from patient-specific iPS cells to search for seed compounds that may lead to new therapies one day. Taking a different approach, we are also examining whether we can exploit genome editing to repair genetic mutations as a therapeutic strategy for muscular dystrophy. If this approach proves effective, we will aim to develop novel therapies based on genome editing.