
News & Events
News & Events
News
March 11, 2020
A simple synthetic tool to activate translation and build RNA circuits
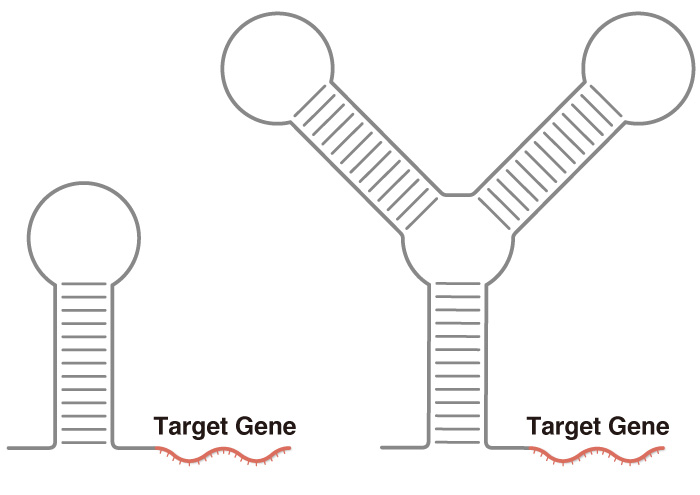
Direct translation activation requires less bulk to the synthetic RNA (left) than indirect activation through translation repression (right).
CiRA Professor Hirohide Saito and scientist Dr. Hideyuki Nakanashi report about Caliciviral VPg-based translational activator, or CaVT, for the construction of synthetic translational regulators and gene circuits in human cells. CaVT is the first synthetic tool to directly activate the translation of RNA. This ability simplifies the construction of all-RNA synthetic gene circuits, which normally operate through translational repression. The study shows that CaVT can be used to induce apoptosis and edit genes in targeted cells, and to activate translation by small molecules.
Synthetic gene circuits can modify cell behavior arbitrarily, allowing scientists to change, for example, a cancerous cell into a dead one. Most gene circuits are constituted of DNA. DNA is long-lasting, which is an attractive feature if the desired effect should be permanent, but could also be dangerous if the circuit incorporates itself incorrectly into the cell's endogenous DNA.
"RNA gene circuits are transient, so they can be used to control cells in a safe way" says Saito, which is why his lab has devoted itself to creating all-RNA gene circuits.
In general, although all-RNA gene circuits control the translation of proteins, they operate by suppressing translation. Thus, to activate the translation of a desires protein, the translation of other protein inhibitors is suppressed. This adds bulk and complexity to the design.
"There are many ways to indirectly activate the translation of RNA circuits. However, indirect translational activation is complicated and requires more components, which adds burden to the cell. The best system is a simple system," explains Nakanishi.
CaVT is constituted of VPg, a viral protein that promotes RNA translation, and MS2CP, a commonly used RNA-binding protein. By modifying the CaVT RNA-target, the researchers could induce the death of specific cells. Furthermore, the level of translation could be modified through strategic engineering of the location, sequence and nucleosides of the motif.
Even more, a single CaVT could also regulate differently multiple target proteins simultaneously, promoting the activation of one and the suppression of the other.
"We can modulate the translational level in opposite directions by engineering the locations of the RNA-binding motif and the affinity between CaVT and the mRNA," says Saito.
In addition, CaVT could be used to control the CRISPR-Cas9 system for targeted gene editing and could also be activated by drugs, giving it the whole gambit of functions seen in all-RNA gene circuits that act by translational suppression.
"One of the major goals of gene circuits is medical therapies. RNA systems are preferred because they do not cause insertional mutagenesis and only weak immune reactions. Simple components like CaVT make it easier to build circuits with specific and strong effects," says Saito.
Paper Details
- Journal: Nature Communications
- Title: Caliciviral protein-based artificial translational activator for mammalian gene circuits with RNA-only delivery
- Authors: Hideyuki Nakanishi* and Hirohide Saito
*current employment: Tokyo Medical Dental University - Author Affiliations:
Department of Life Science Frontiers, Center for iPS Cell Research and Application, Kyoto University, Kyoto, Japan






















