
News & Events
News & Events
News
March 25, 2020
Clinical research for the transfer of autologous iPS cell-derived platelets to a thrombocytopenia patient
Kyoto University Hospital and CiRA is conducting a clinical research in which platelets derived from the iPS cells of a patient with aplastic anemia*1 complicated by platelet transfusion refractoriness*2 will be transfused into the same patient. Here the collaborators announce that the patient has completed the dosing schedule. To protect the privacy of the patient, no more information is to be given at this time.
One of the outcomes of aplastic anemia is a low platelet count. Although patient transfusion is one treatment, in some patients the platelet count does not rise, and they develop a refractoriness to the transfusion. One reason is that the transfused platelets elicits an immune response. To minimize an immune reaction, platelets using the patient's own cells are recommended.
| May 28, 2018 | Plan for the clinical research checked by the Kyoto University Regenerative Medicine Evaluation Subcommittee |
|---|---|
| July 20, 2012 | Plan for the clinical research submitted to the Ministry of Health, Labour and Welfare (MHLW) |
| August 29, 2018 | MHLW conducts its first deliberation of the plan |
| September 21, 2018 | MHLW conducts its second deliberation of the plan |
| October 19, 2018 | The plan is approved by MHLW |
| March 25, 2019 | The clinical research begins |
| May 2019 | The first platelet dose is administered |
| August 2019 | The second platelet dose is administered |
| January 2020 | The third platelet dose is administered |
1. Name of plan
Clinical research for the transfer of autologous iPS cell-derived platelets to treat thrombocytopenia
2. Purpose
To verify the safety of the autologous transfusion of platelets made from the peripheral blood mononuclear cells (PBMCs)*3 reprogrammed into iPS cells of a patient with aplastic anemia complicated by platelet transfusion refractoriness.
3. Experimental design
Single-center, open-label, uncontrolled study.
The patient received three dose cohorts
a. Cohort 1: 0.5 units (1*1010 platelets)
b. Cohort 1: 1.5 units (3*1010 platelets)
c. Cohort 1: 5.0 units (1*1011 platelets)
4. Target disease
Aplastic anemia complicated by platelet transfusion refractoriness
5. Number of patients
One
6. Follow-up period
One year from the time of the final transfusion
7. Cell process
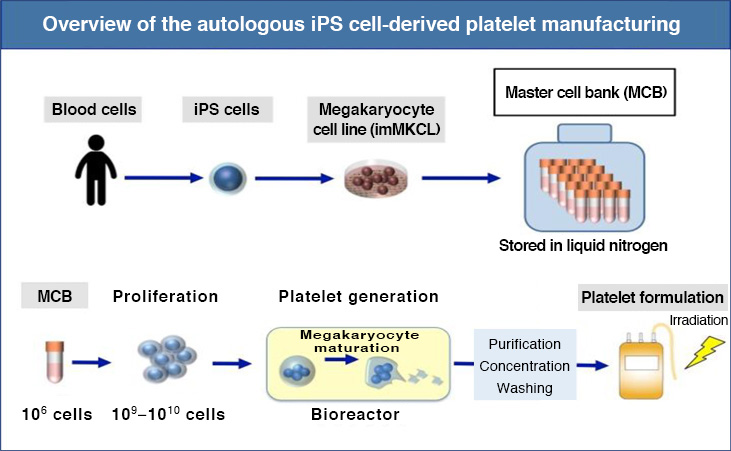
8. PBMCs were collected from the patient and reprogrammed into iPS cells. The iPS cells were then differentiated into a megakaryocyte cell line*4 that was stored frozen as a master cell line*5. The master cell line was thawed, proliferated, and differentiated into platelets. The platelets were purified, concentrated, washed, irradiated and finally transfused into the patient.
9. Endpoints
Primary: safety (frequency and extent of adverse events)
Secondary: efficacy (platelet count*6)
1) Aplastic anemia
A rare disease (approximately 8 per million people) in which the count of red blood cells, white blood cells and platelets are all reduced.
2) Platelet transfusion refractoriness
A condition where the platelet count does not increase even after a transfusion. Causes normally involve an immune reaction to the transfused platelets.
3) Peripheral blood mononuclear cells
A set of leukocytes that includes lymphocytes and monocytes.
4) Megakaryocyte cell line
Megakaryocytes are a unique type of blood cell in that when they mature, they undergo nuclear division but not cell division, resulting in large, multinucleated cells. The megakaryocyte cell line used in this project is derived from genetically modified iPS cells, so that the megakaryocytes can be matured and proliferated arbitrarily.
5) Master cell line
A starting-cell source for manufacturing the final cell product.
6) Platelet count
This count considers the patient body size (surface area) and is an indicator of the effectiveness of the transfusion.
-
Research and development (manufacturing)
Prof. Koji Eto, Dept. of Clinical Application, CiRA -
Chief Physician
Prof. Akifumi Takaori, Dept. of Hematology and Oncology, Graduate School of Medicine, Kyoto University -
Hospital Unit
Dept. of Hematology and Oncology, Kyoto University Hospital - Collaborating Institutes CiRA (production and quality control from the iPS cell stage to the platelet stage) and the Institute for Advancement of Clinical Translational Science (IACT), Kyoto University (preparation and implementation of clinical research)
This research was funded by the Japan Agency for Medical Research and Development.






















