
News & Events
News & Events
News
December 01, 2023
Developing more advanced renal organoids to model polycystic kidney disease
Autosomal dominant polycystic kidney disease (ADPKD) affects about 1 in 1,000 to 4,000 people worldwide. While most patients can typically live a normal life, advanced stages of the disease may require dialysis or transplantation. While some potentially useful medications, such as Tolvaptan, are available to help reduce cyst growth, it remains a genetic condition without any cure treatments.
Kidney organoids generated from human induced pluripotent stem cells (hiPSCs) represent a powerful biological tool that can provide us with a better understanding of the disease mechanism and a means to model diseases for drug discovery and testing purposes. However, organoids often remain in a developmental stage and do not accurately model disease conditions in adults. For ADPKD, organoid models have been previously reported, but none could reach the maturation status required to express key molecules involved in collecting duct cyst formation. As a result, spontaneous cyst formation has never been observed in such models, so kidney organoids generated to date have yet to demonstrate their full potential for drug discovery and testing.
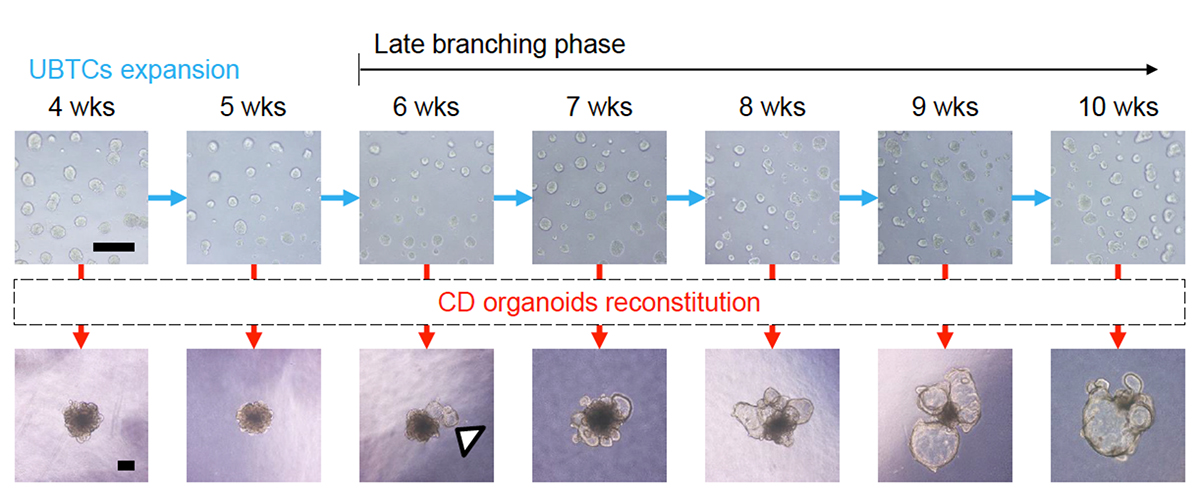
In a recent study led by Professor Kenji Osafune (Department of Cell Growth and Differentiation), a team of researchers successfully generated cortical cell-containing collecting duct organoids by establishing a method for long-term expansion culture of ureteric bud tip cells (UBTC) to reach the maturity required to model ADPKD accurately. The team, noting that TGF-β inhibition promotes branching and the population in kidney organoids, started by modifying the differentiation protocol they previously reported by incorporating A83-01, a TGF-β inhibitor, into the culture medium and extended culture duration from 2 weeks to 6 weeks. By performing RNA sequencing on kidney organoids with or without A83-01 treatment and comparing their gene expression profiles, the researchers identified and verified, by chemical inhibition, the NFκB pathway as vital to UBTC proliferation.
Using these more mature UBTCs, the research team proceeded to induce collecting duct formation through a one-week differentiation process. Although collecting duct principal cells can be generated from UBTCs in culture for 2 or 6 weeks, long-term cultured UBTCs (6 weeks) showed a gene expression profile consistent with a more developed, late branching phase not displayed by short-term cultured UBTCs. Furthermore, inflammatory response and cellular senescence-related markers were upregulated in the more mature UBTCs, consistent with a gradual switch toward slower growth. Next, the researchers focused on collecting duct organoids generated from UBTCs cultured for 2 or 6 weeks and identified by single-cell RNA sequencing inner medullary collecting duct (IMCD) cells, papillary tip epithelial cells, outer medullary collecting duct (OMCD) principal cells, cortical collecting duct (CCD) principal cells and connecting tubule cells. Notably, a comprehensive comparison of these and previously reported organoids revealed that while CCD cells were not present in organoids from the previous report, they were in the organoids generated in this study, with a slightly higher proportion of this cell type in organoids differentiated from long-term cultured UBTCs. In-depth analysis by the researchers identified additional changes in gene expression that indicate a more developmentally advanced status for these organoids.
To determine whether humans and mice share similar renal developmental mechanisms and if these collecting duct organoids could be used to model ADPKD, the researchers generated homozygous PKD1 KO hiPSCs by CRISPR/Cas9 because Pkd1 KO mice develop cysts during embryonic development. The team remarkably observed spontaneous cyst formation in PKD1 KO but not wild-type (WT) control collecting duct organoids. Furthermore, the researchers found these spontaneously formed cysts to respond to manipulations (e.g., cyclic AMP signaling and cholesterol production) believed to affect cyst formation, thus demonstrating they accurately recapitulate their in vivo counterparts.
To determine the utility of these collecting duct organoids for high-throughput screening to identify small molecules potentially useful for ADPKD therapy, the researchers established a 96-well plate-based platform and tested several drug candidates currently being examined in clinical trials (e.g., venglustat, bardoxolone methyl, and tesevatinib) and tolvaptan, an FDA-approved medication for ADPKD, and found that they were effective in suppressing cyst enlargement and/or formation. The researchers notably observed TTNPB, a retinoic acid receptor (RAR) agonist, to suppress cyst enlargement while establishing the drug screening platform. In addition, all-trans retinoic acid (ATRA) was found in parallel to have similar effects, thus confirming RAR signaling as a putative therapeutic target for ADPKD. Microarray analysis of cyst-forming collecting duct organoids treated with or without TTNPB revealed the potential involvement of multiple signaling pathways, including TGF-β, cholesterol synthesis, glucose transport and glycolysis, and cellular senescence. Focusing on cellular senescence and the upregulation of CDKN2B by TTNPB, the researchers generated an hiPSC line with inducible CDKN2B expression and found cyst enlargement to be attenuated by its overexpression, thus highlighting it as a viable drug target for AKPKD therapy.
Since ATRA is already in clinical use for treating acute promyelocytic leukemia, the research team tested its efficacy in a mouse model of AKPKD (kidney-specific Pkd1 KO mice), which develops severe renal cystic disease, lethal about 2 weeks after birth. Significant qualitative and quantitative improvements were detected in the kidneys of ATRA-treated mice, thus warranting further preclinical and clinical studies for ATRA and other RAR agonists for ADPKD treatment.
Remarkably, the modified methods for long-term expansion culture and differentiation to generate more mature cortical cell-containing collecting duct organoids open the door for various basic research and clinical applications. As demonstrated by the research team in this study, they possess enormous potential for studies to deepen our understanding of ADPKD pathogenesis and treatment and to function as a robust platform for drug discovery and testing purposes.
Paper Details
- Journal: Cell Reports
- Title: Human iPSC-derived renal collecting duct organoids model cystogenesis in ADPKD
- Authors:
Shin-Ichi Mae1, Fumihiko Hattanda2, Hiroyoshi Morita1, Aya Nozaki1, Naoko Katagiri1,
Hanako Ogawa3, Kaori Teranaka3, Yu Nishimura3, Aoi Kudoh4, Sanae Yamanaka5, Kyoko Matsuse1, Makoto Ryosaka1, Akira Watanabe3,4, Tomoyoshi Soga5, Saori Nishio2, Kenji Osafune1*
*:Corresponding authors - Author Affiliations:
- Center for iPS Cell Research and Application (CiRA), Kyoto University
- Department of Rheumatology, Endocrinology and Nephrology, Faculty of Medicine and Graduate School of Medicine, Hokkaido University
- CyberomiX Co., Ltd.
- Medical Innovation Center, Graduate School of Medicine, Kyoto University
- Institute for Advanced Bioscience, Keio University






















