
News & Events
News & Events
News
April 16, 2024
Optimizing differentiation protocols and experimental assays to study patient-specific astrocytes
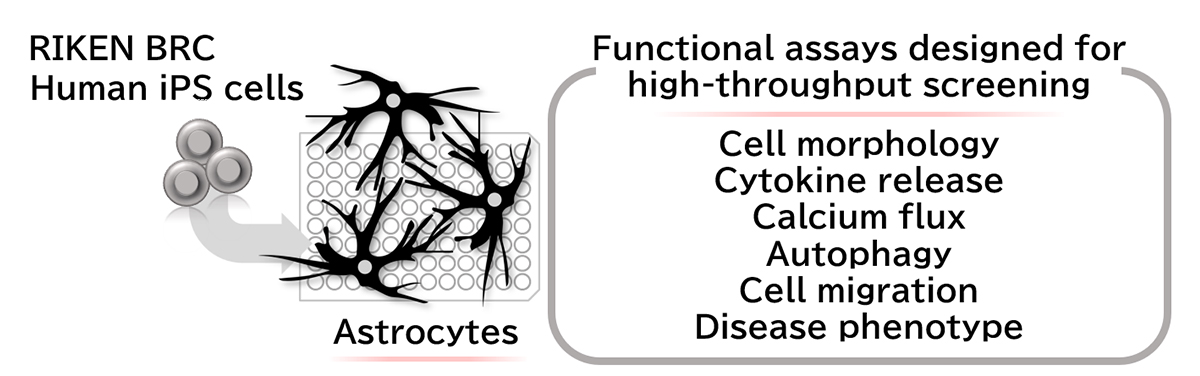
Astrocytes, one of two major types of supporting cells in the central nervous system (i.e., brain and spinal cord), are increasingly recognized for their involvement in many human diseases including stroke and Alzheimer's disease. As such, it is critical to determine how they are affected in the pathophysiological state and whether they could be targeted for treatment and cure.
The research team had previously established a "feeder-free" protocol to generate astrocytes from iPS cells (iPSCs), thus enabling disease modeling and drug discovery. However, establishing high-throughput screening platforms based on human iPSC-derived astrocytes requires robust and reproducible functional assays with the potential for large-scale operations. Since such experimental pipelines to analyze various astrocytic functions are currently unavailable, therapeutic development using iPSC-derived astrocytes has been limited. Therefore, in this study, the researchers primarily focused on validating several functional assays designed to assess vital biological processes that astrocytes are involved in and could dramatically impact disease progression: cytokine release, calcium flux, autophagy, and cell migration.
Neuroinflammation, particularly the release of inflammatory cytokines from astrocytes, can be detrimental to brain cells during disease progression or following injuries. Therefore, the researchers determined whether iPSC-derived astrocytes are responsive to inflammatory stimuli such as tumor necrosis factor-alpha (TNF-α) and interleukin 1-beta (IL-1β). Following TNF-α or IL-1β treatment, the research team detected cytokines released into the media from iPSC-derived astrocytes, thus demonstrating their responsiveness to inflammatory stimulation.
Astrocytic calcium homeostasis is especially crucial to neuronal functions and survival. The researchers examined whether iPSC-derived astrocytes can undergo ATP-induced calcium uptake and detected dose-dependent increases in calcium influx of the astrocytes.
Autophagy, one of the principal degradative pathways in cells, is enhanced by nutrient starvation and often compromised in neurodegenerative diseases, not only in neurons but also in astrocytes. The researchers measured autophagy using a fluorescent probe in normal and starvation conditions and observed an increase in autophagy in iPSC-derived astrocytes after starvation, reduced when it was chemically blocked.
Astrocytes migrate to lesion sites to mediate wound healing and tissue repair in response to traumatic injuries and other stimuli. Because these processes can drastically affect functional recovery, the research team also assessed whether iPSC-derived astrocytes properly migrate in response to stimulation. Through the scratch assay, in which cells are scratched off the surface of a cell culture plate, they observed both iPSC-derived astrocytes and primary astrocytes to similarly migrate into the injured region.
Lastly, having confirmed that iPSC-derived astrocytes behave similarly to primary astrocytes, the research team next generated astrocytes from two iPSC lines, one established from a healthy volunteer and another from an Alexander disease (AxD) patient carrying a mutation in the GFAP gene. The researchers observed a significant increase in GFAP-positive aggregates in AxD iPSC-derived astrocytes.
In summary, the research team validated iPSC-derived astrocytes to function similarly to primary astrocytes. Crucially, their work reveals the potential of using iPSC-derived astrocytes as an in vitro model of disease or injury. Furthermore, the assays examined by the researchers are ripe for adaptation to establish high-throughput screening pipelines for identifying chemical or genetic factors affecting astrocytic functions under patient-specific disease conditions. Altogether, the series of experiments in this study paves the way forward for drug discovery against various astrocyte-associated pathologies using iPSC-derived astrocytes.
Paper Details
- Journal: Journal of Cellular and Molecular Medicine
- Title: Induced pluripotent stem cell-based assays recapture multiple properties of human astrocytes
- Authors:
Hideki Nonaka1,2,**, Takayuki Kondo1,3,4,**, Mika Suga1,3, Ryu Yamanaka2, Yukako Sagara1,3, Kayoko Tsukita1,3, Naoko Mitsutomi2, Kengo Homma2, Ryuta Saito2, Fumihiko Miyoshi2, Hiromitsu Ohzeki2, Masahiro Okuyama2, Haruhisa Inoue1,3,4,*
**: Equally contributed to this work
*: Corresponding author - Author Affiliations:
- iPSC-based Drug Discovery and Development Team, RIKEN BioResource Research Center (BRC)
- Mitsubishi Tanabe Pharma Corporation
- Center for iPS Cell Research and Application (CiRA), Kyoto University
- Medical-risk Avoidance based on iPS Cells Team, RIKEN Center for Advanced Intelligence Project (AIP)






















