
News & Events
News & Events
News
April 24, 2024
Elucidating pathogenic mechanisms underlying α-synucleinopathy-related dementia using patient iPSC-derived neurons
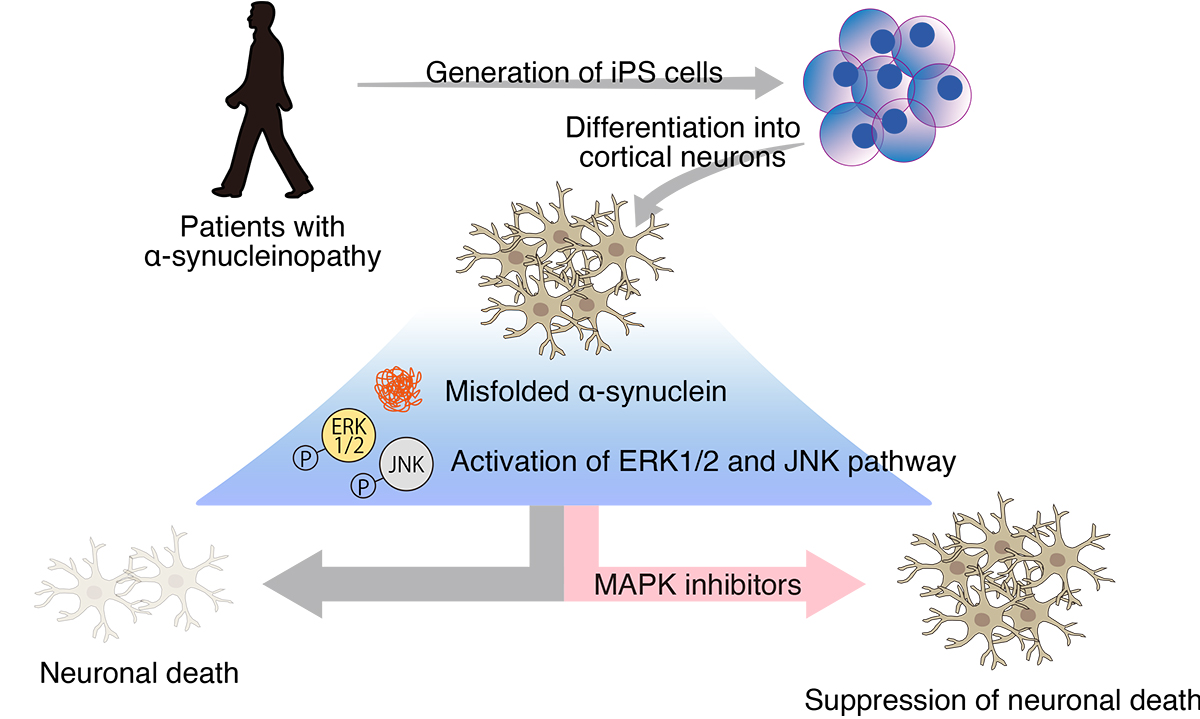
α-Synucleinopathies encompass a group of neurological diseases such as PD and dementia with Lewy bodies characterized by the pathogenic accumulation and aggregation of α-synuclein. These diseases affect different brain regions leading to motor, cognitive, and behavioural deficits.
The research team first established several independent iPSC lines from two patients with the A53T mutation in the SNCA gene, associated with familial PD, and three healthy volunteers. Next, the researchers differentiated those iPSCs into cortical excitatory neurons with a commonly used direct conversion protocol. After confirming the generation of mature cortical neurons, the researchers observed increased α-synuclein accumulation and aggregation in α-synucleinopathy patient iPSC-derived neurons. Conversely, reduced neurite length and increased apoptosis were observed in α-synucleinopathy neurons, suggesting mutant α-synuclein reduced neuronal health and viability.
To investigate the responsible pathological mechanisms in detail, the research team compared the gene expression profile of healthy and α-synucleinopathy neurons by RNA sequencing. Notably, the transcriptomes of independently derived α-synucleinopathy neurons showed high similarity and were distinct from the health neurons. The expression of over 150 genes was significantly altered between healthy and α-synucleinopathy neurons. Correspondingly, bioinformatic analysis suggested various biological processes were likely dysregulated in α-synucleinopathy neurons. In particular, the MAP kinase pathway was predicted to be upregulated and may be involved with the observed reduced neurite length and enhanced neurotoxicity. The researchers thus focused on the MAP kinase pathway by examining key proteins involved in its regulation and their activation status. Remarkably, whereas p38 was mostly unaltered in α-synucleinopathy neurons, the researchers found ERK1/2 and JNK to be hyperactivated in α-synucleinopathy neurons. To assess whether the aberrant activation of ERK1/2 and JNK pathways is involved in the reduced viability of α-synucleinopathy neurons, the research team treated α-synucleinopathy neurons with varying doses of PD98059 or SP600125, selective inhibitors against ERK1/2 and JNK, respectively, and examined for apoptotic activation. Remarkably, ERK1/2 or JNK inhibition was observed to significantly reduce caspase-3/7 activation, a hallmark of apoptosis, by nearly 50%, thus suggesting that the pathologic activation of these two MAP kinases in α-synucleinopathy neurons contributes substantially to their demise.
This study was the first to connect aberrant ERK1/2 and JNK overactivation and increase cell death of α-synucleinopathy cortical neurons. Previous animal studies have suggested the involvement of those pathways in the neurodegeneration occurring in the nigrostriatal pathways. However, since most animal α-synucleinopathy models do not fully recapitulate the human pathology, cortical alterations are rarely observed. Therefore, the research team could better model certain aspects of α-synucleinopathy pathology by using patient-specific iPSC-derived cortical neurons. Hopefully, the research team will be able to expand on this work and create a high-throughput screening platform based on patient-specific iPSC-derived cortical neurons to perform chemical and genetic screens and identify previously unknown disease modifiers as potential new drugs and therapeutic targets.
Paper Details
- Journal: Molecular Brain
- Title: Mutant α-synuclein causes death of human cortical neurons via ERK1/2 and JNK activation
-
Authors: Hidefumi Suzuki1,2,3, Naohiro Egawa1,2,3, Keiko Imamura2,3,4, Takayuki Kondo2,3,4, Takako Enami3,4, Kayoko Tsukita2,3, Mika Suga2,3, Yuichiro Yada2,3, Ran Shibukawa2, Ryosuke Takahashi1,*, Haruhisa Inoue2,3,4,*
*: Corresponding authors - Author Affiliations:
- Department of Neurology, Graduate School of Medicine, Kyoto University
- iPSC-Based Drug Discovery and Development Team, RIKEN BioResource Research Center (BRC)
- Center for iPS Cell Research and Application (CiRA), Kyoto University
- Medical-Risk Avoidance Based on iPS Cells Team, RIKEN Center for Advanced Intelligence Project (AIP)






















