CiRA Reporter
CiRA Reporter

Focus
April 21, 2023
New Form of Drug Discovery Made Possible by iPS Cells (Part 1)

Haruhisa Inoue
How is "iPS drug discovery" conducted?
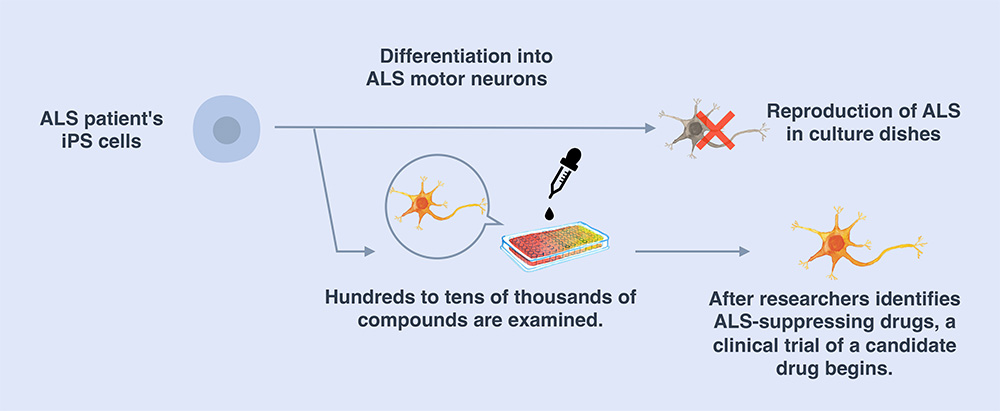
We create iPS cells from the patient's somatic cells and turn them into the target diseased cells. These "disease model cells" are compared with healthy cells in experiments to identify the differences between them and healthy cells. In addition, we can use the disease model cells to screen for substances that can be used as drugs to restore them back to health. In other words, the newly identified substances have the potential to be candidate drugs for new treatments. From there, we proceed to clinical trials. This whole process is what we call “iPS drug discovery.”
You are currently working on iPS drug discovery for ALS (amyotrophic lateral sclerosis)* patients. Could you tell us more about this project?
To date, there is no viable treatment for ALS. We are employing the iPS drug discovery process to study drugs for ALS. In 2008, we generated iPS cells for the first time from a patient with hereditary ALS using the technology for generating human iPS cells developed in 2007. From those iPS cells, we generated degenerative motor neurons with the same genetics as ALS patients. Meanwhile, we also developed a new method to produce large quantities of motor neurons with high consistency.
We found that motor neurons from ALS patients tend to die more quickly in culture dishes than motor neurons from healthy individuals. We used these cells as disease model cells and examined the effects of about 1,400 compounds, one by one, including drugs already used for treating other diseases or drugs that are under development. As a result, 27 drugs strongly suppressed motor neuron death. Half of these compounds inhibited the function of the protein Src/c-Abl. Thus, through iPS drug discovery, we were able to establish a novel druggable target for ALS and identified candidate drugs that act via this new target. After reporting this work in 2012 and 2017, we prepared for clinical trials and started the “Phase 1 clinical trial of bosutinib for amyotrophic lateral sclerosis (ALS)” in 2019.
"iPS drug discovery" using patients' iPS cells to identify therapeutic drugs (e.g., ALS)
Your team is currently conducting a clinical trial for ALS patients, in cooperation with other clinical institutes. What kind of results has been obtained?
Bosutinib, the drug being tested in this clinical trial for ALS patients, is used to treat chronic myeloid leukemia. Adverse events such as diarrhea and liver dysfunction were observed throughout the trial, but they were not specific to ALS patients. During the course of treatment with the investigational drug, there were indications that ALS progression was halted in some patients. In the next phase of the clinical trial, with an increased number of subjects, we will continue to examine the safety and efficacy of the drug.
You are currently developing an AI to search for effective compounds for drug discovery. Can you tell us about this project?
The probability of successful drug discovery through compound screening (the process of selecting effective compounds from a large number of drug candidate compounds) can be enhanced by finding drug seeds from as many compounds as possible and selecting the best ones. Takeda Pharmaceutical Co., Ltd. ("Takeda"), which is our partner in the T-CiRA joint research program, holds one of the world's largest compound libraries and we have searched through approximately 1.6 million compounds in their library to identify potential therapeutic candidates for ALS. The process to screen through this vast number of compounds is both time- and cost-intensive, so we narrowed down the number of compounds to 5,600, with the help of AI, the heat diffusion equation model, and finally found five compounds to be ideal seeds for developing new ALS drug candidates. Today, research on those compounds continues at Takeda.
How did you become involved in iPS drug discovery?
I was originally a neurologist and took care of many ALS patients. In the 1990s, when some ALS causative genes had just been identified and ALS model mice were being developed, I started working on finding a treatment, but translating this research from animals to humans would take an enormous amount of time. I was hoping to find information directly from ALS patients, so I tried to create skin stem cells from ALS patients to find drugs, but things didn’t go well.
At that time, Dr. Shinya Yamanaka succeeded in producing iPS cells. I thought that if I could generate iPS cells from ALS patients' skin cells to create disease model cells, it would be possible to identify effective therapeutic drugs through iPS drug discovery, so I decided to apply for a position at CiRA.
What do you think of the potential for drug development using iPS cells?
iPS cell technology enables to test the actions of drugs under conditions that most closely resemble the patients’. It can be used to validate a wide range of therapeutic drug candidates, including small molecule compounds, and nucleic acid- or antibody-based therapeutic agents. In the future, if it becomes feasible to create iPS cells from every patient, we will be able to examine disease model cells for each individual and achieve the ultimate goal of personalized medicine.
* ALS (amyotrophic lateral sclerosis): A disease of unknown cause in which motor neurons selectively degenerate and die. Muscle atrophy and weakness are the main symptoms, which consequently cause dysfunctions of the upper limbs, abnormal gait, and problems with swallowing and breathing as the disease progresses. The pathogenic mechanisms are not fully understood, and there are still no treatment options available presently.
* The Japanese version of this article was published in CiRA Newsletter Vol.52.
Interview and article by Akihico Mori
(Translation: CiRA International Public Communications Office, Research Promoting Office)






















