CiRA Reporter
CiRA Reporter

Focus
August 08, 2023
iPS Cells: The Past Ten Years and the Future
As the Central Hub of iPS Cell Research in Japan
In 2013, the Ministry of Education, Culture, Sports, Science and Technology (MEXT) announced it would provide long-term support of 110 billion yen over 10 years for regenerative medicine research in Japan. The "Research Center Network for Realization of Regenerative Medicine" was supported by this funding and focused on research using iPS cells and related technology for regenerative medicine, as well as research elucidating the mechanisms of disease and creating new medicines. Universities, research institutions, and private companies from all over Japan participated in the program, from basic to applied research and non-clinical trials. Research groups working on ethical issues and regulatory compliance necessary to realize new medical treatments also participated.
CiRA researchers also participated in various research projects. There are two major streams of medical applications for iPS cells: (1) regenerative medicine, which uses cells created from iPS cells to replace lost functions, and (2) investigation of disease mechanisms and novel drugs using iPS cells. In both research directions, CiRA has been playing a central role in Japan.
iPS Cells for Studying Disease Mechanisms
iPS cells derived from patients have the potential to reproduce the disease conditions inside their bodies. CiRA has generated and deposited iPS cells from patients with various diseases at the RIKEN BioResource Center so that they are readily available to researchers. To date, iPS cells for 231 different diseases have been made (Reference: List of disease-specific iPS cells at RIKEN BioResource Center). While there are other facilities that store similar iPS cell lines worldwide, this is considered the largest collection of disease-specific iPS cells.
Clinical Trials of Drugs Found Using iPS Cells
In Japan, five clinical studies have been conducted to evaluate the safety and efficacy of new drug candidates discovered using disease-specific iPS cells by administering them to patients. The candidate compounds tested in three of these studies were discovered by research groups at CiRA.
| Start year | Target disease | Candidate substance | Reference | ||
|---|---|---|---|---|---|
| 2017 | Start year: 2017 |
Fibrodysplasia Ossificans Progressiva (FOP) |
Target disease: Fibrodysplasia Ossificans Progressiva (FOP) Candidate substance: Sirolimus |
Sirolimus | |
| 2018 | Start year: 2018 | Pendred Syndrome |
Target disease: Pendred Syndrome
Candidate substance: Sirolimus |
Sirolimus | |
| 2018 | Start year: 2018 |
Amyotrophic Lateral Sclerosis (ALS) |
Target disease: Amyotrophic Lateral Sclerosis (ALS) Candidate substance: Ropinirole Hydrochloride |
Ropinirole Hydrochloride | |
| 2019 | Start year: 2019 |
Amyotrophic Lateral Sclerosis (ALS) |
Target disease: Amyotrophic Lateral Sclerosis (ALS) Candidate substance: Bosutinib |
Bosutinib | |
| 2020 | Start year: 2020 | Alzheimer's Disease |
Target disease: Alzheimer's Disease
Candidate substance: Bromocriptine |
Bromocriptine |
Although these drugs have yet to be approved for clinical use, it is hoped that they will be delivered to patients as new medicines in the near future.
In addition, efforts are being made to encourage the use of disease-specific iPS cells to conduct research on more diseases and to facilitate further research collaborations.
Production of iPS Cells for Regenerative Medicine
CiRA played the most significant role in the Core Center of iPS Cell Research, a program supporting a wide range of research activities, ranging from basic research on how iPS cell reprogramming works and how to create them with increased efficiency and quality to applied clinical research on how to produce clinical grade “iPS Cell Stock for Regenerative Medicine.” Sharing scientific information with other research institutes working to develop iPS cell-based therapies, CiRA manufactured and provided iPS cells for medical use to research institutions in need. The CiRA Foundation has taken over the production of iPS cells for regenerative medicine since 2020.
More Than 10 Clinical Trials using iPS Cell Stock Initiated
Research is also underway to develop treatments from iPS cell stocks by transforming them into the needed cells. A project aimed at treating Parkinson's disease headed by Professor Jun Takahashi at CiRA began a clinical trial in 2018 (CiRA News, July 30, 2018). The transplantation procedure was completed for seven patients by the end of 2021, and the patients have been monitored for two years. The results of the clinical trial concerning the safety and efficacy of those cell transplants will be disclosed in the near future.
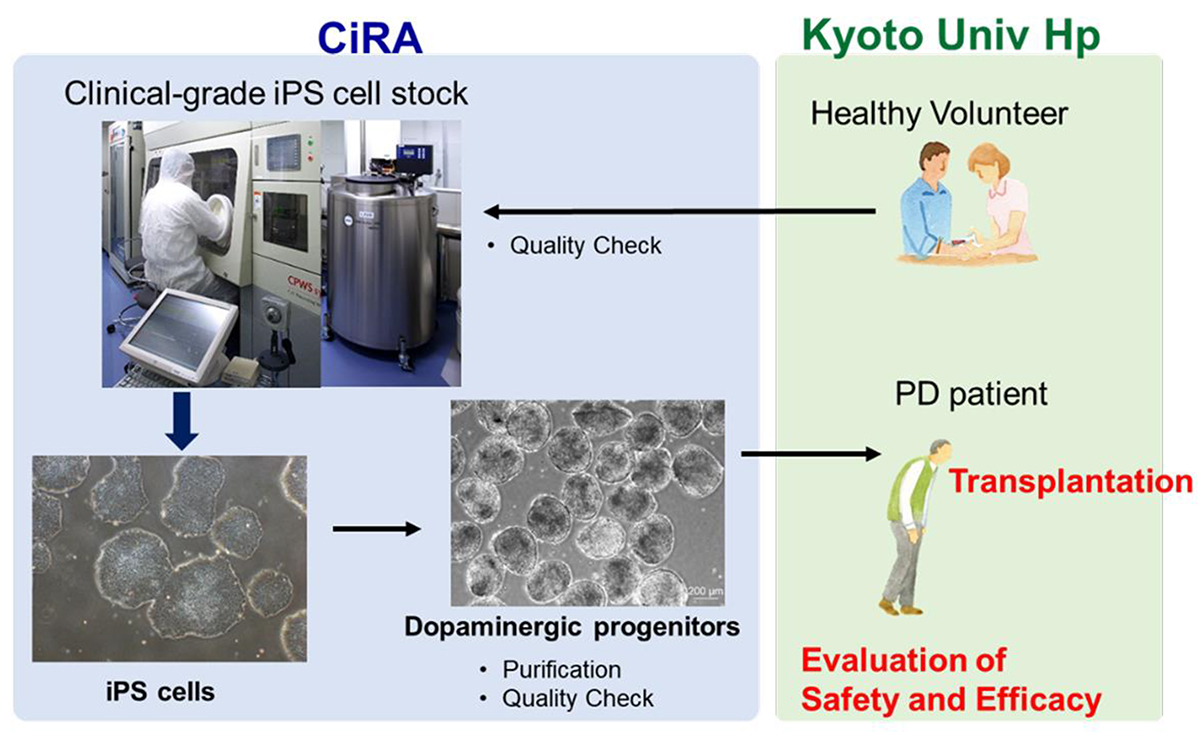
Clinical trials for Parkinson's diseases
As of April 2023, 17 clinical trials have started in Japan to verify the safety and efficacy of transplanting cells created from iPS cells into patients. Of these, 14 projects used iPS cell stocks generated by CiRA or the CiRA Foundation. None of them has advanced to become approved medical treatments yet, but they have been moving steadily toward that primary goal. Hopefully, some will soon become treatment options available to patients.
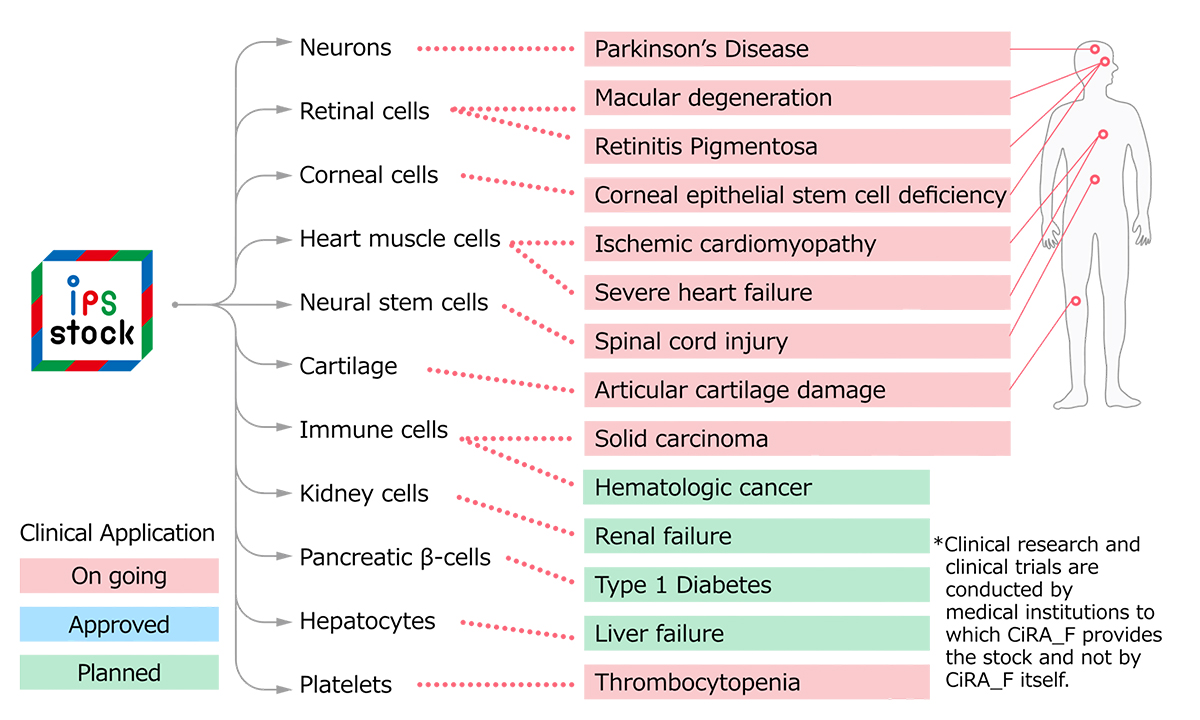
Clinical trials using iPS cell stock
(as of April 2023)
Source: CiRA Foundation website
*In some cases, multiple projects are being conducted for the same disease.
*In some cases, multiple projects are being conducted for the same disease.
Toward A Future of New Medicine
Based on research using iPS cell technology, new treatments for various diseases are being created. However, this still only represents a small fraction of all human diseases. Moreover, new therapies are not necessary cures, so they alone may not solve all of a patient’s health problems.


Jun Takahashi
CiRA Director Jun Takahashi says, "Regenerative medicine is, so to speak, a comprehensive art. Simply transplanting cells into patients does not cure their disease. We must look to combine cell transplantation with other therapeutic approaches, like drug and gene therapies, medical devices, and rehabilitation, and develop new techniques and equipment for culturing cells and innovative systems for providing medical care. Only by amalgamating the wisdom and power of multiple research disciplines will we be able to provide treatments best suited for individual patients."
Research into therapies that combine cell transplantation and gene therapy is making exciting progress. (CiRA News, April 6, 2023). As the CiRA Foundation began providing HLA genome-edited iPS cell stock for clinical use to research institutes in June of this year, it is now possible to provide iPS cell-based therapies to more patients than ever.
From here onward, we can look forward to novel treatment methods created by using iPS cells as invaluable tools and integrating research from various fields, not limiting ourselves to only the fruits of fusing cell and gene therapy.
- Written by Hiroyuki Wadahama Ph.D.
Science Communicator, CiRA International Public Communications Office






















