
Research Activities
Research Activities
Publications
November 28, 2016
High-sensitivity neurotoxicity assay of anti-cancer drugs
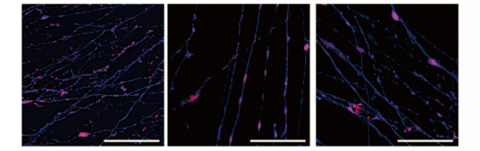
Vincristine, an anti-cancer drug, caused mitochondria (red) to accumulate in neurons made from CMT patient iPS cells (middle and right). In cells made from healthy volunteers (left), no accumulation occurs
Before a drug is made available to the public, it must pass rigorous tests that affirm its safety. Drugs designed to kill cells, like anti-cancer drugs (ACD), can have severe toxic effects on non-cancerous cells, which is why a great deal of research is invested in assays that evaluate drug toxicity.
Neurons are especially sensitive to ACD, but human neurons are difficult to acquire, therefore many scientists depend on animal models. Because of their pluripotency and availability, iPS cells can be differentiated into neurons to provide a human model for drug toxicity assays. Furthermore, because iPS cells can be acquired from patients, more reliable tests on patient cells can be made.
The Haruhisa Inoue laboratory at CiRA took advantage of these properties to investigate the toxicity of ACD in Charcot Marie Tooth disease (CMT). "CMT patients express severe side effects with anti-cancer drugs," said Assistant Professor Keiko Imamura, a neurologist in the Inoue lab. "Genetically, they are sensitive to anti-cancer drugs, so we used their cells to test the neurotoxicity."
Among neurological disorders, CMT is one of the most commonly inherited, and patients show dysaesthiasia (i.e. abnormal sensitivity to pain) along with growth deformities and eventual muscle atrophy due to neurodegeneration.
The Inoue team hypothesized that because CMT patients show severe neuropathy after the administration of ACD, their iPS cells could be used to prepare a highly sensitive assay for drug neurotoxicity.
Indeed, neurotoxicity assays revealed that the toxicity of two ACDs, vincristine and paclitaxel, on both iPS cell-derived neurons from CMT patients and normal volunteers resulted in mitochondrial aggregates that foreshadowed neuronal death, but the effect was stronger in the CMT patients.
Inoue believes these findings indicate how the assay can pinpoint the cause of the neurotoxicity. "We see many phenotypes in drug neurotoxicity, but we don't know which is an appropriate assay for prediction of the toxicity," he said. These results suggest ACD neurotoxicity may be exasperated if there are any genetic predispositions and that the assay presented by the Inoue lab could be used to identify vulnerable patients.
Paper Details
- Journal: Clinical Pharmacology and Therapeutics
- Title: Modeling drug-induced neuropathy using human iPSCs for predictive toxicology
- Authors: Ryo Ohara1,2, Keiko Imamura1, Fukiko Morii1,2, Naohiro Egawa1, Kayoko Tsukita1, Takako Enami1, Ran Shibukawa1, Toshiki Mizuno2, Masanori Nakagawa2,3, and Haruhisa Inoue1
- Author Affiliations:
- Center for iPS Cell Research and Application (CiRA), Kyoto University, Kyoto, Japan
- Department of Neurology, Kyoto Prefectural University of Medicine, Kyoto, Japan
- North Medical Center, Kyoto Prefectural University of Medicine, Kyoto, Japan






















