Research Activities
Research Activities
Principal Investigators
Dept. of Life Science Frontiers
Knut Woltjen (Associate Professor)

Knut Woltjen Ph.D.
Research Overview
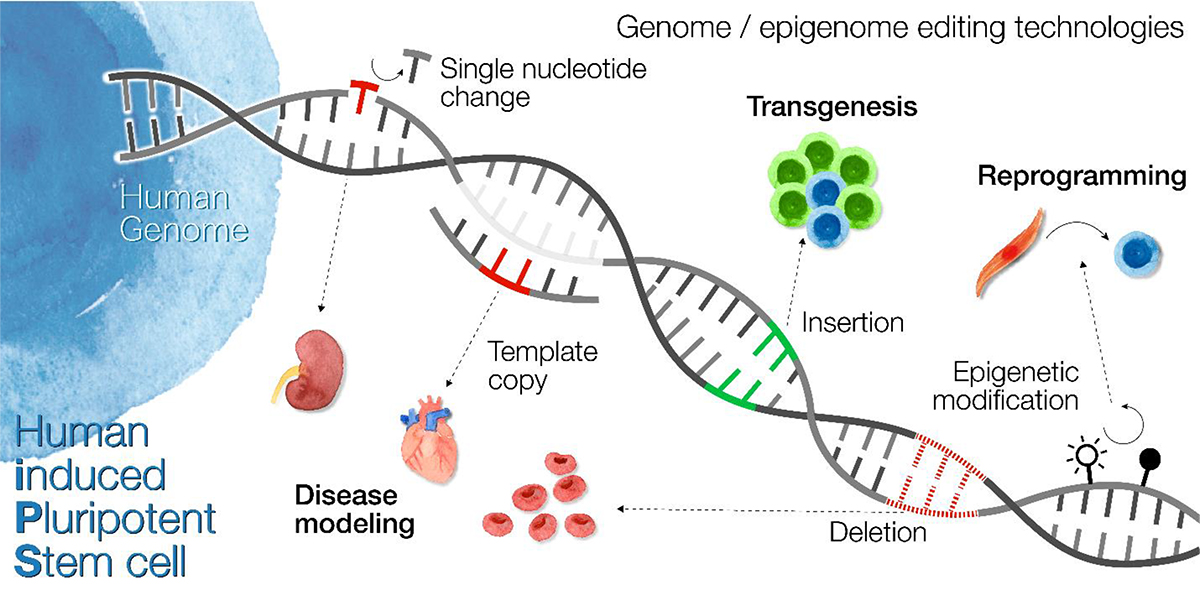
About thirty-five years after the Human Genome Project began, we now live in an era of widespread human genome sequencing. Yet, most functions encoded in the human genome remain mysterious. Human induced pluripotent stem (iPS) cells maintain the same genome as their donor and gain the capacity to differentiate into cell types affected by genetic disease. Therefore, iPS cells represent a powerful approach to functional genomics and personalized medicine.
The Woltjen Lab specializes in cutting-edge gene editing technologies in iPS cells to achieve "total control of the human genome." Our goal is to understand the genome and to develop and apply techniques to correct mutations and introduce new gene functions. To this end, we have developed methods to precisely edit single-nucleotides and create deletions, the two most common classes of pathogenic human genetic variants. Furthermore, we are establishing a precise editing method for repetitive DNA sequences which are highly variable throughout human evolution and amongst individuals. By addressing these critical challenges, our research addresses significant technical hurdles in the field to understand genome function and its therapeutic potential.