News & Events
News & Events
News
December 06, 2023
Establishing a revolutionary model to study early human embryonic development
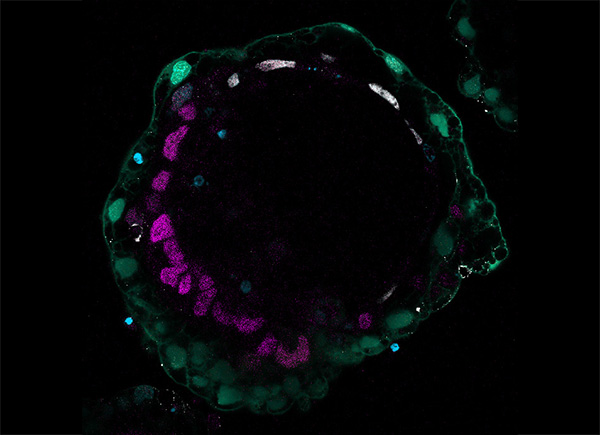
Immunofluorescence image of a bilaminoid
Our knowledge of the early stages of human embryonic development, during implantation into the uterine endometrium, remains limited, partly due to ethical and legal issues, despite its importance to basic developmental biology, reproductive health, and regenerative medicine. While scientists have learned much from experimental animal models, it remains unclear how much of the derived mechanistic details apply to humans due to potential species differences. Recently, several research teams from around the world have announced human stem cell-based embryo models that provide a window to observe developmental events typically hidden in the uterus. Nonetheless, by mimicking the developmental phase following implantation, these models effectively skipped the implantation barrier to model post-implantation embryonic development. Therefore, there remains a fundamental knowledge gap in early human development because current models have yet to recapitulate the natural developmental sequence and timing from pre-implantation to peri-gastrulation.
To explore an even earlier point in the human developmental timeline previously inaccessible to scientists, the researchers induced GATA6, GATA4, or SOX17 transgene expression in naïve or primed embryonic stem cells (ESCs) based on the knowledge that those genes can initiate hypoblast formation. While SOX17 failed to induce hypoblast marker genes, GATA6 and, to a lesser extent, GATA4, overexpression triggered hypoblast formation. Critically, comprehensive gene expression analysis showed that GATA6 overexpression in naïve and primed ESCs produced substantially different cell populations: whereas naïve ESCs were nudged into the hypoblast fate, primed ESCs assumed a post-implantation stage, like in the recently reported stem cell-based embryo models. Through detailed analysis of the gene expression changes in these naïve ESCs, the researchers deduced signaling pathways crucial for specification into hypoblasts. They attempted to activate or inhibit those same pathways chemically and successfully identified a combination of 7 factors that induced ES and iPS cell lines into hypoblast cells of the pre-implantation stage.
Equipped with genetic and chemical approaches to generate hypoblast cells, the research team aimed to model peri-implantation development that intertwines their newly generated hypoblasts and PSCs. They observed the cell mixture to form aggregates-called bilaminoids by the researchers-grow in cell number and self-organize, with the hypoblast cells relocating to the edge. Gene expression analysis consistently showed a progression of both tissues into a late human blastocyst post-implantation state. The bilaminoids notably began forming pro-amniotic-like cavities, bilaminar disc, anterior-posterior axis, and a basement membrane separating the hypoblast and epiblast. The researchers also observed the emergence of cell types indicative of lineage specification in the bilaminoids by the end of their experiments, further suggesting maturation to a post-implantation-like stage. They further found that epiblast proliferation and amniotic cavity formation were enhanced by co-culturing bilaminoids with trophoblast-like cells, highlighting the support role of this second extraembryonic tissue during peri-implantation development.
Crucially, because the researchers developed this model system by separately inducing and culturing the three cell types that make up the blastocyst (i.e., epiblast, hypoblast, and trophoblast), they were able to manipulate them independently for mechanistic studies to decipher how their interactions regulate embryonic development. For instance, the researchers demonstrated the requirement for trophoblast-secreted IL6 to be responsible for the trophoblast-mediated enhancement in bilaminoid growth and cavitation by deleting the IL6 gene in naïve PSCs used to generate trophectoderm. As another example, the researchers deleted the LAMB1 gene (encoding the laminin subunit beta 1 protein) from naïve PSCs before turning them into hypoblasts and discovered the resulting bilaminoids to be devoid of the basement membrane separating the hypoblast and epiblast and the pro-amniotic cavity, therefore highlighting the importance laminins in epiblast differentiation and morphogenesis.
This study was the first to demonstrate robust and reproducible genetic or chemical induction of pre-implantation hypoblast-like cells, which the research team used to produce a human stem cell-based peri-implantation embryo model. The researchers demonstrated the utility of their new model system by investigating different aspects of peri-implantation embryonic development and dissecting interactions between the different cell lineages that were never before possible. Their breakthrough perfectly complements the recently reported post-implantation embryo models to help clarify our understanding of early human embryonic development. Without a doubt, these embryo model systems together will propel human developmental research into a new age.
Paper Details
- Journal: Nature
- Title: Hypoblast from human pluripotent stem cells regulates epiblast development
- Authors:
Takumi Okubo1, Nicolas Rivron2, Mio Kabata1, Hideki Masaki3,4, Keiko Kishimoto5,
Katsunori Semi1, May Nakajima-Koyama1, Haruko Kunitomi1, Belinda Kaswandy1, Hideyuki Sato3,4, Hiromitsu Nakauchi3,4,6, Knut Woltjen1, Mitinori Saitou1,7,8, Erika Sasaki5, Takuya Yamamoto1,7,9,*,
Yasuhiro Takashima1,*
*:Corresponding authors - Author Affiliations:
- Center for iPS Cell Research and Application, Kyoto University
- Institute of Molecular Bioatactechnology of the Austrian Academy of Sciences
- Institute of Medical Science, University of Tokyo
- Advanced Research Institute, Tokyo Medical and Dental University
- Central Institute for Experimental Animals
- Stanford University School of Medicine
- Institute for the Advanced Study of Human Biology (WPI-ASHBi)
- Department of Anatomy and Cell Biology, Graduate School of Medicine, Kyoto University
- Medical-risk Avoidance based on iPS Cells Team, RIKEN Center for Advanced Intelligence Project (AIP)