
News & Events
News & Events
News
July 02, 2025
Laying the foundation for future jawbone research
The jawbone, consisting of maxillary and mandibular bones, is the largest biomineralized organ in the oral region and is highly susceptible to irreversible damage caused by various pathogenic factors, including genetic defects, infections, and trauma. However, a model to recapitulate jawbone development and pathophysiology has yet to be established. The research team led by Ikeya recently accomplished this feat, thus laying the groundwork for future studies investigating jawbones and developing therapies for conditions that affect them.
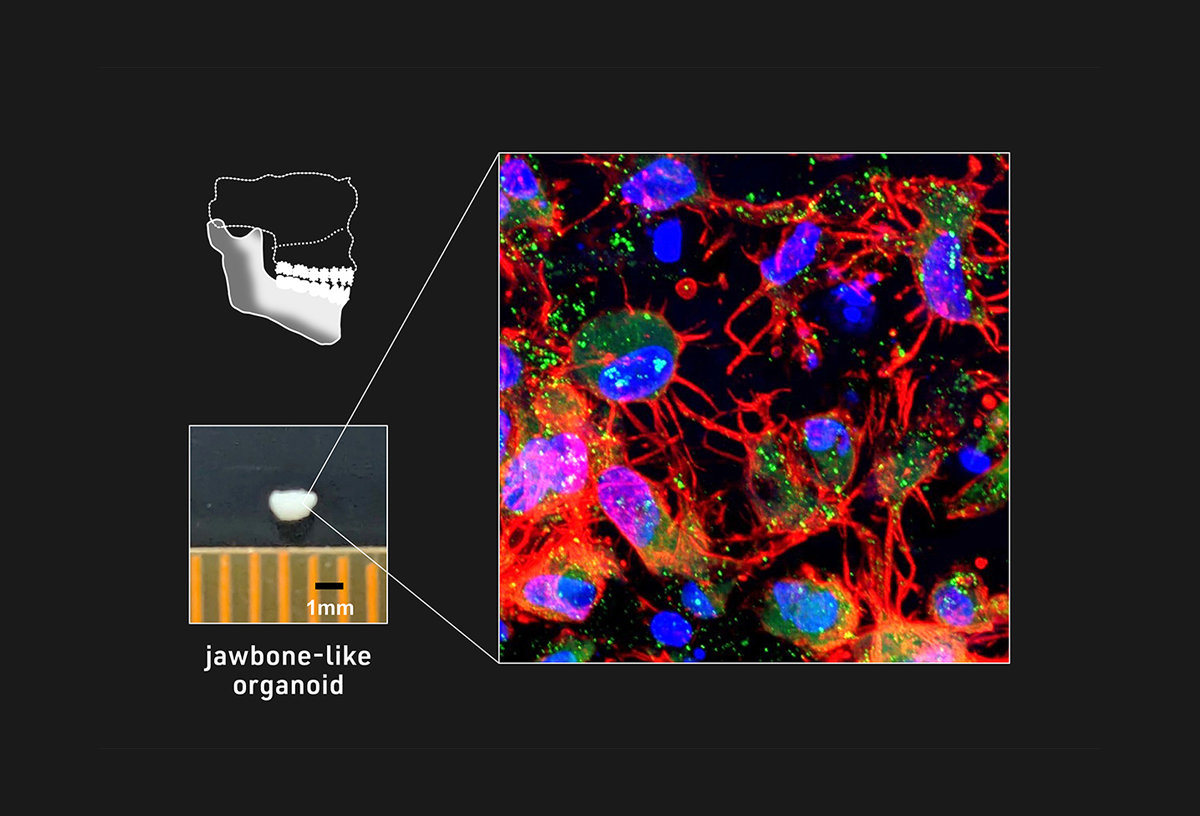
The researchers previously established the means to generate, in two-dimensional (2D) cell culture, the specific type of cranial neural crest cells (NCCs) capable of differentiating into cells that make up the jawbone. Based on that know-how, the research team aimed to modify their protocol to induce three-dimensional (3D) jawbone organoids. After optimizing various conditions, including fine-tuning cell signaling pathways and cell culture apparatus, they were able to efficiently induce the desired NCC subtype and grow them as cell aggregates using multiple independent iPS cell lines, including those derived from patients with osteogenesis imperfecta (OI), an inherited bone disorder also known as brittle bone disease. Using hints from embryonic development, the researchers identified a combination of molecules to stimulate NCC aggregates into an intermediate cell type called mandibular prominence ectomesenchyme (mdEM). Detailed analyses and additional treatment of these iPS cell-derived mdEMs revealed that they resemble embryonic mdEMs and could be further induced into more differentiated tissues.
Next, the researchers cultured iPS cell-derived mdEMs in osteo-inductive conditions and obtained white, mineralized tissue clusters with various markers and characteristics of bone formation, thus suggesting the successful generation of jawbone-like organoids capable of constructing bone tissue. Hypothesizing that these organoids represent an early stage of jawbone development before the formation of a blood supply, they transplanted iPS cell-derived mdEMs under the capillary-rich renal capsules of immunocompromised mice (to avoid an immune response against the grafted tissue) and observed the formation of highly mineralized and vascularized mature bone tissues. Then, to evaluate the regenerative capacity of these jawbone-like organoids, they were transplanted directly into artificial mandibular jawbone defects in immunocompromised mice. Although the mineralized tissue density was not as high as that of bone-grafted mice, animals with organoid grafts showed a similar increase in mineralized tissue, which closely resembled mature bone tissue and was vascularized. These findings together indicate that jawbone-like organoids could promote bone regeneration following transplantation.
Since the research team also generated NCCs using iPS cells from an OI patient with an autosomal dominant mutation in the COL1A1 gene, they questioned whether they could use jawbone-like organoids derived from these NCCs and their genetically rescued counterparts to model OI pathophysiology. After a few days of inducing bone formation from COL1A1 mutant mdEMs, the secretion of aberrant collagen was detected. In addition, gene expression analysis revealed that COL1A1 and various markers of osteogenic cells were significantly reduced in OI-iPS cell-derived jawbone-like organoids. Histological and morphological analyses further unveiled immature bone matrices and abnormal bone-forming cells. By contrast, all these pathological features were ameliorated in genetically rescued OI-iPS cell-derived jawbone-like organoids. Lastly, mutant and rescued organoids were transplanted under renal capsules of immunocompromised mice to examine their in vivo bone-forming potential. As expected, OI-iPS cell-derived jawbone-like organoids led to reduced bone tissues, with many characteristics typical of OI bones. Furthermore, mutant bone-forming cells showed increased signs of cell death, along with disoriented bone matrices, resembling the disease.
Through this effort, the research team successfully generated jawbone-like organoids representing a fundamental research advance that will benefit research on human jawbone embryonic development, pathophysiology, and therapeutic development.
Paper Details
- Journal: Nature Biomedical Engineering
- Title: Jawbone-like organoids generated from human pluripotent stem cells
- Authors: Souta Motoike1, Yoshiko Inada1, Junya Toguchida1, Mikihito Kajiya2, Makoto Ikeya1*
*: corresponding author - Author Affiliations:
- Center for iPS Cell Research and Application (CiRA), Kyoto University
- Hiroshima University Hospital






















