
News & Events
News & Events
News
July 17, 2025
From dormancy to healing: An iPSC blueprint to generate mature heart organoids for epicardial reactivation
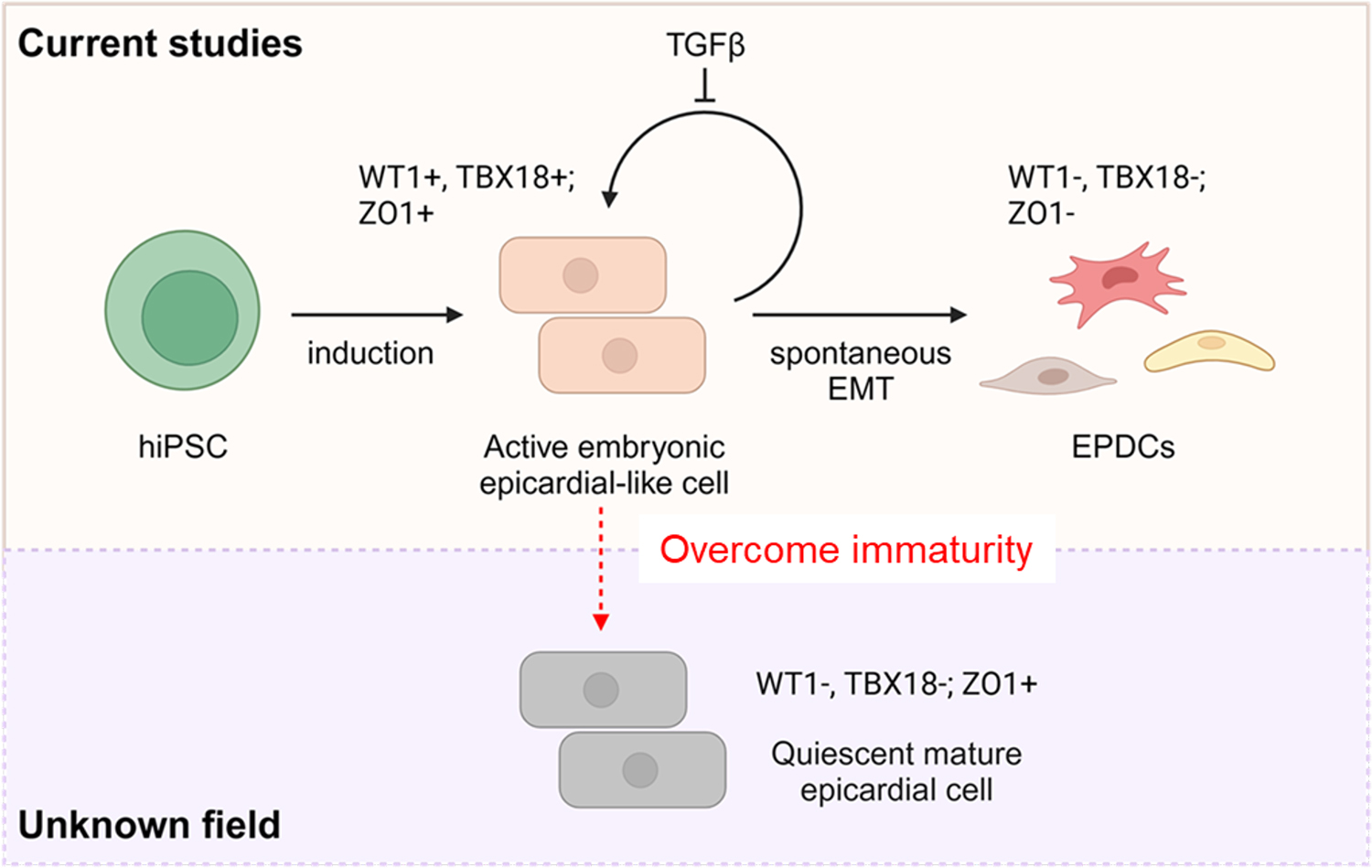
Modulating the mTOR pathway has emerged as a key developmental cue for driving functional maturation and quiescence in the human epicardium. Leveraging this insight, a team led by CiRA Associate Professor Yoshinori Yoshida (Department of Growth and Differentiation) and Antonio Lucena‑Cacace, Associate Professor at Osaka University, has created a first‑class cellular platform comprising mature epicardium and mature heart organoids. This system allows researchers to explore how quiescent epicardial tissue can be reactivated during cardiac repair.
Successful cardiac regeneration - repopulating damaged tissue and restoring physiologically compatible function - demands exquisitely coordinated signaling across multiple heart layers, including the epicardium. While this outermost layer contributes extensively to cardiac architecture and function in utero, it provides little regenerative support after birth. In humans, the post‑natal epicardium remains largely refractory to proliferation, even when injury signals call for assistance. Previous attempts to generate mature epicardium in vitro have progressed slowly, largely because the developmental cues underlying its non‑proliferative state were poorly understood.
Now, by placing induced pluripotent stem (iPS)‑cell technology at the forefront once again, the team has shown that inhibiting mTOR, a nutrient‑sensing pathway that intersects with proliferation, development, homeostasis, and aging, triggers timely epicardial maturation.
"As stem cell biologists, we can now toggle epicardial proliferation and maturation almost at will," notes Yu Tian, researcher and co first author of the study. "Watching cells physiologically exit the cell cycle deepens our understanding of how similar processes might be harnessed to regenerate the human heart," she said. "It is incredibly rewarding to identify small molecules finally capable of reinstating proliferation in dormant tissue. This represents a significant step toward future clinical translation."
To test whether mTOR modulation could be extended from epicardial sheets to whole cardiac tissue, Tian, Lucena‑Cacace, and colleagues generated three‑dimensional, self‑assembling heart organoids from iPS cells. A brief mTOR‑inhibition "pulse" yielded more mature organoids with clear signs of neovascularization. The team further assessed epicardial monolayers in a high‑throughput chemical screen, identifying several small molecules, most notably GSK3 inhibitors, that show promise for re‑awakening quiescent epicardium.
"We are witnessing the convergence of mature organoid systems, iPS‑cell technology, large language models, and AI," says Professor Yoshida, who led the study. "This intersection will propel the next generation of regenerative therapies, and our lab is thrilled to be part of it."
The newly developed organoids and epicardial tissues constitute a powerful tool for pinpointing compounds that can trigger regenerative responses in preclinical models, opening an entirely fresh perspective on cardiac repair.
Lucena‑Cacace, the study's co‑senior author, also underscores its impact: "Highly mature organoids and epicardium provide ideal training data for AI‑driven models that predict how to reinstate controlled proliferation. With multiple reports of vascularized heart organoids now emerging, our platform further elevates the state of the art and strengthens future cardiac‑therapy pipelines."
Published in Nature Communications, this discovery establishes a robust "heart‑in‑a‑dish" framework and places iPS cells once more at the forefront of in‑vitro systems for studying cardiac development, disease modelling, and regenerative interventions.
Paper Details
- Journal: Nature Communications
- Title: Generation of mature epicardium derived from human-induced pluripotent stem cells via inhibition of mTOR signaling
- Authors:
Yu Tian1,2†, Antonio Lucena-Cacace1,3*†, Kanae Tani1,2, Amanda Putri Elvandari1, Rodolfo S. Allendes Osorio3, Megumi Narita1, Yasuko Matsumura1, Ian Costa Paixao1, Yutaro Miyoshi1,2, Azusa Inagaki1, Julia Junghof1,2, Yoshinori Yoshida1*
†: These authors contributed equally (co-first)
*: Corresponding authors - Author Affiliations:
- Center for iPS Cell Research and Application (CiRA), Kyoto University
- Graduate School of Medicine, Kyoto University
- Premium Research Institute for Human Metaverse Medicine (WPI-PRIMe), Osaka University






















