Research Activities
Research Activities
Principal Investigators
Dept. of Cell Growth and Differentiation
Yoshinori Yoshida (Associate Professor)

Yoshinori Yoshida M.D., Ph.D.
- Lab Website
- Research Progress in FY2024
- Contact: yoshida-g*cira.kyoto-u.ac.jp
- Please change * to @
Research Overview
Currently, our lab is using iPS cells to study heart cells with application towards regenerative medicine, drug discovery, and disease modeling. If you are interested, please contact us.
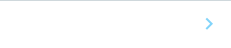
1. Development of myocardial regenerative medicine
Regenerative medicine is critical for patients with limited treatment options. We are using iPS cells to seek new therapies for this purpose.
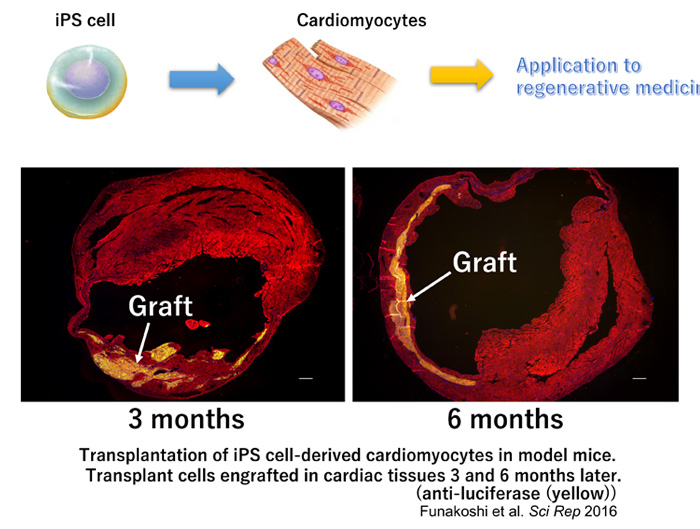
2. Disease-specific iPS cells
iPS cells are invaluable for disease models, as they can be designed to recapitulate disease phenotypes in vitro. We use iPS cells to study cardiac diseases (e.g., cardiomyopathies). Furthermore, we use gene editing technology, such as CRISPR/Cas9, to modify iPS cells from diseased patients. We then differentiate edited and unedited iPS patient cells into the cell type of interest to identify phenotypic differences. Our laboratory aims to analyze disease mechanisms and develop therapeutic drugs using iPS cells established from the cells of patients with cardiac diseases (cardiomyopathy, cardiac arrhythmias, etc.).
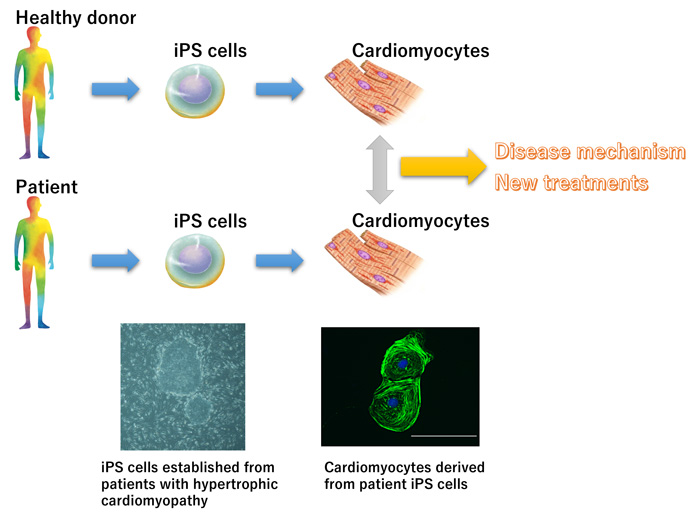
3. Cardiomyocytes for clinical application
Cardiomyocytes and tissues created from iPS cells are being utilized as alternatives to animal model-based drug discovery and drug toxicity testing. Cardiomyocytes include various subtypes, such as pacemaker, atrial, and ventricular cells. Each subtype has its role in the normal and diseased heart. Another factor besides the subtype is the maturation of cardiomyocytes. Therefore, we investigate protocols that differentiate iPS cells into the proper subtypes and maturity to study related diseases and novel therapies.
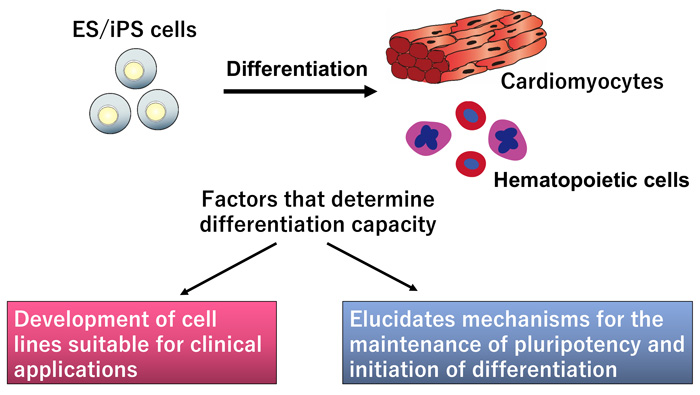
4. Investigation into mechanisms determining the differentiation capacity of ES/iPS cells
ES/iPS cell clones show variable differentiation capacities into different somatic cell types. We are investigating the mechanisms that govern these differences by comparing the behavior of cell lines during their differentiation into cardiomyocytes. The goal of this project is to establish iPS cell clones suitable for clinical applications.
|
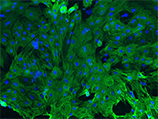
Cardiomyocytes derived from human iPS cells
(Green: cardiac isoform of Troponin T, Blue: DAPI) |
|
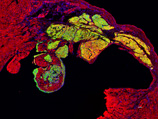
| Cardiomyocytes derived from human iPS cells provide a transplant for myocardial infarction mouse (Yellow) |
|
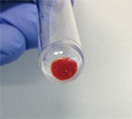
| Hematopoietic cells derived from human iPS cells |
|