
News & Events
News & Events
News
July 24, 2025
Streamlining differentiation with non-destructive AI-based imaging
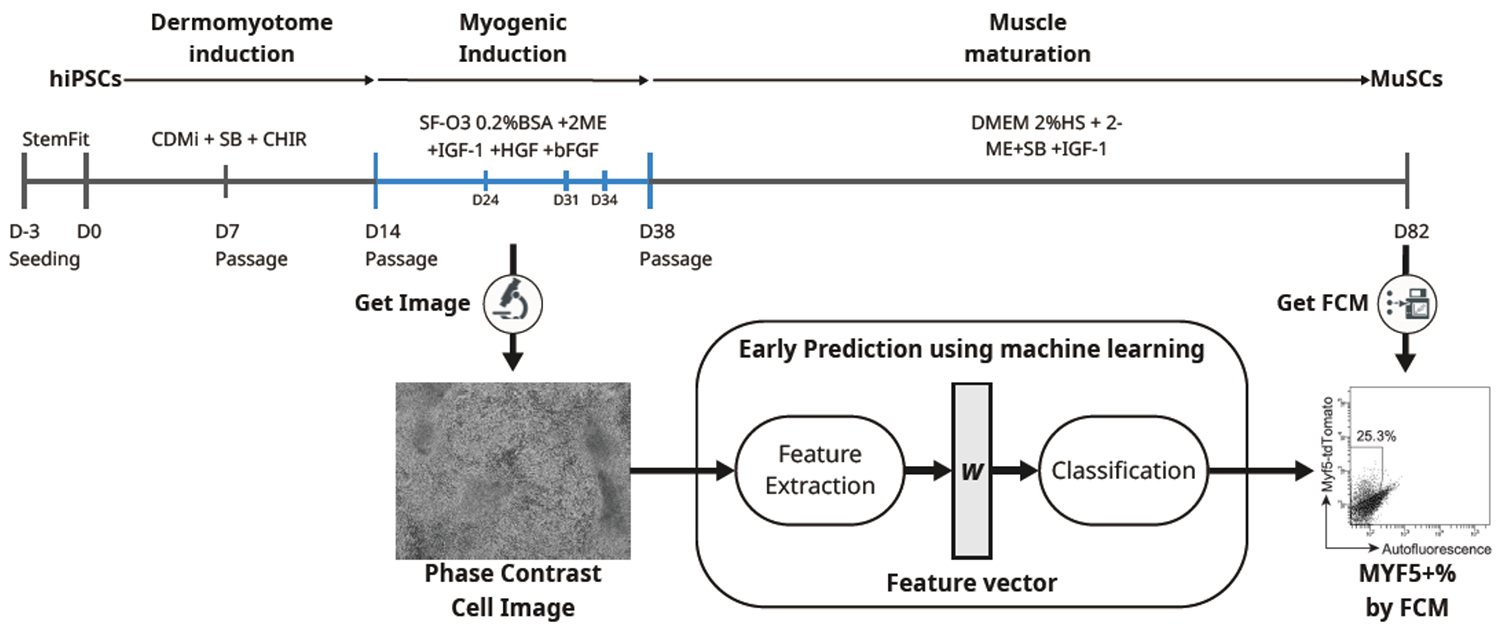
Human iPS cells present great promise for regenerative medicine due to their ability to differentiate into essentially all cell types in the human body. Among the various differentiation strategies, directed differentiation is widely used because it avoids genetic manipulation and mimics natural developmental processes. However, such protocols often suffer from low reproducibility and require long induction periods, making it difficult to optimize conditions and select high-quality samples early. This study addresses that challenge by introducing a system that combines phase contrast imaging with machine learning to forecast the final differentiation efficiency of iPS cells into muscle stem cells (MuSCs), a key cell type for treating muscular dystrophies.
To develop this predictive system, the research team employed a previously established MuSC induction protocol and collected over 5,500 phase contrast images from 34 wells between days 14 and 38 of differentiation. Using fast Fourier transform (FFT), they extracted morphological features from those images and trained a random forest classifier to predict the percentage of MYF5-positive cells—a marker of successful MuSC differentiation—on day 82. The system demonstrated high predictive accuracy, particularly when using images from day 24 to identify low-efficiency samples and days 31 or 34 to identify high-efficiency ones.
Biological validation supported the imaging-based predictions. Gene and protein expression levels of myogenic markers such as MYH3 and MYOD1 on day 38 showed a strong correlation with final differentiation outcomes. Immunocytochemistry confirmed that samples with higher expression of these markers tended to yield more MYF5-positive cells. The classifier reduced the proportion of low-quality samples by 43.7% and increased the yield of high-quality ones by 72%, demonstrating its practical value in streamlining cell production.
Importantly, the system is non-invasive and does not require destructive assays, which are typically labor-intensive and unsuitable for real-time monitoring. By enabling early and objective assessment of differentiation potential, this method offers a powerful tool for improving the reproducibility and efficiency of iPS cell-based protocols. It also reduces the time, reagent, and labor costs associated with long-term cell culture and analysis, making it highly applicable to both research and clinical manufacturing settings.
The study highlights the potential of applying machine learning to image analysis to enhance stem cell research and production. The team now plans to explore the applicability of this approach to other differentiation systems, which could accelerate the development of stem cell-based therapies across a range of diseases and contribute to the broader advancement of regenerative medicine.
Paper Details
- Journal: Scientific Reports
- Title: Early and non-destructive prediction of the differentiation efficiency of human induced pluripotent stem cells using imaging and machinelearning
- Authors:
Miki Arai Hojo1*, Taku Tsuzuki2, Yosuke Ozawa2*, Toshiyuki Araki3, and Hidetoshi Sakurai1*
*: Corresponding author - Author Affiliations:
- Center for iPS Cell Research and Application (CiRA), Kyoto University
- Epistra Inc.
- National Center of Neurology and Psychiatry (NCNP)






















