
News & Events
News & Events
News
September 08, 2025
Development of a highly efficient method for generating iPS cells from human peripheral blood cells

Graphical abstract
Created with BioRender.com
Key Findings
-
Successful reprogramming of PBMCs using synthetic RNA
We have successfully established induced pluripotent stem (iPS) cells from peripheral blood mononuclear cells (PBMCs) using synthetic RNA, which has been a technical challenge until now. -
Dramatic improvement in reprogramming efficiency by inhibiting the p53 pathway
Introducing MDM4, a negative regulator of p53, significantly enhanced the reprogramming efficiency of PBMCs using RNA. -
The S367A mutant of MDM4 is the most effective
Among various MDM4 mutants tested, the S367A variant, which is resistant to ubiquitin-mediated degradation, exhibited the highest effectiveness. -
Established PBMC-derived iPS cells can differentiate into corneal cells
The generated iPS cells demonstrated the ability to differentiate into all three germ layers, including successful induction into corneal epithelial-like cells, confirming their pluripotency. -
Protocol publication
The somatic cell (HDF and PBMC) reprogramming protocols, using synthetic RNA, have been published on the CiRA website in both Japanese and English.
Summary
A research group led by Dr. Masato Nakagawa (Junior Associate Professor at the Center for iPS Cell Research and Application (CiRA), Kyoto University, and Specially Appointed Associate Professor at the Premium Research Institute of Human Metaverse Medicine (WPI-PRIMe), The University of Osaka) has established a highly efficient method for generating induced pluripotent stem (iPS) cells from human peripheral blood mononuclear cells (PBMCs) using synthetic RNA. This study offers an effective solution to the longstanding challenge of non-viral iPS cell generation from human blood cells, potentially contributing to future applications in regenerative and personalized medicine. The findings have been published in the open-access journal Scientific Reports.
Background of the Study
iPS cells can be generated from various somatic cells and are widely used in drug discovery, disease modeling, and potentially regenerative medicine. Among these, PBMCs are a highly promising donor source for iPS cell generation due to their minimally invasive and easy collection.
However, while traditional methods using viral vectors are effective, RNA-based reprogramming using synthetic mRNA—a non-viral, non-genome-integrating method—has been considered technically difficult for PBMCs. PBMCs are particularly sensitive to the stress induced by gene delivery, causing substantial cell death and thus poor reprogramming efficiency with the RNA-based method.
Nevertheless, the use of RNA for reprogramming is likely to become a standardized approach due to its safety and the fact that it does not introduce genomic alterations, making it suitable for clinical applications.
Therefore, the objective of this study was to identify the barriers to RNA-based reprogramming of PBMCs and to develop strategies to overcome them. The researchers focused on the hyperactivation of the p53 stress response pathway and investigated whether modulating this pathway could enhance the efficiency of RNA-based reprogramming.
Summary of Research Findings
In this study, the researchers developed a novel method to generate iPS cells from PBMCs using synthetic RNA. Traditionally, PBMCs have shown low reprogramming efficiency, and generating iPS cells using RNA-based methods has been particularly challenging. Our research achieved the following outcomes:
1. Introduction of MDM4, a negative regulator of p53, dramatically improved reprogramming efficiency
In particular, the MDM4 mutant with the S367A substitution, which prevents ubiquitin-mediated degradation, had the highest efficacy, increasing reprogramming efficiency by more than 10-fold.
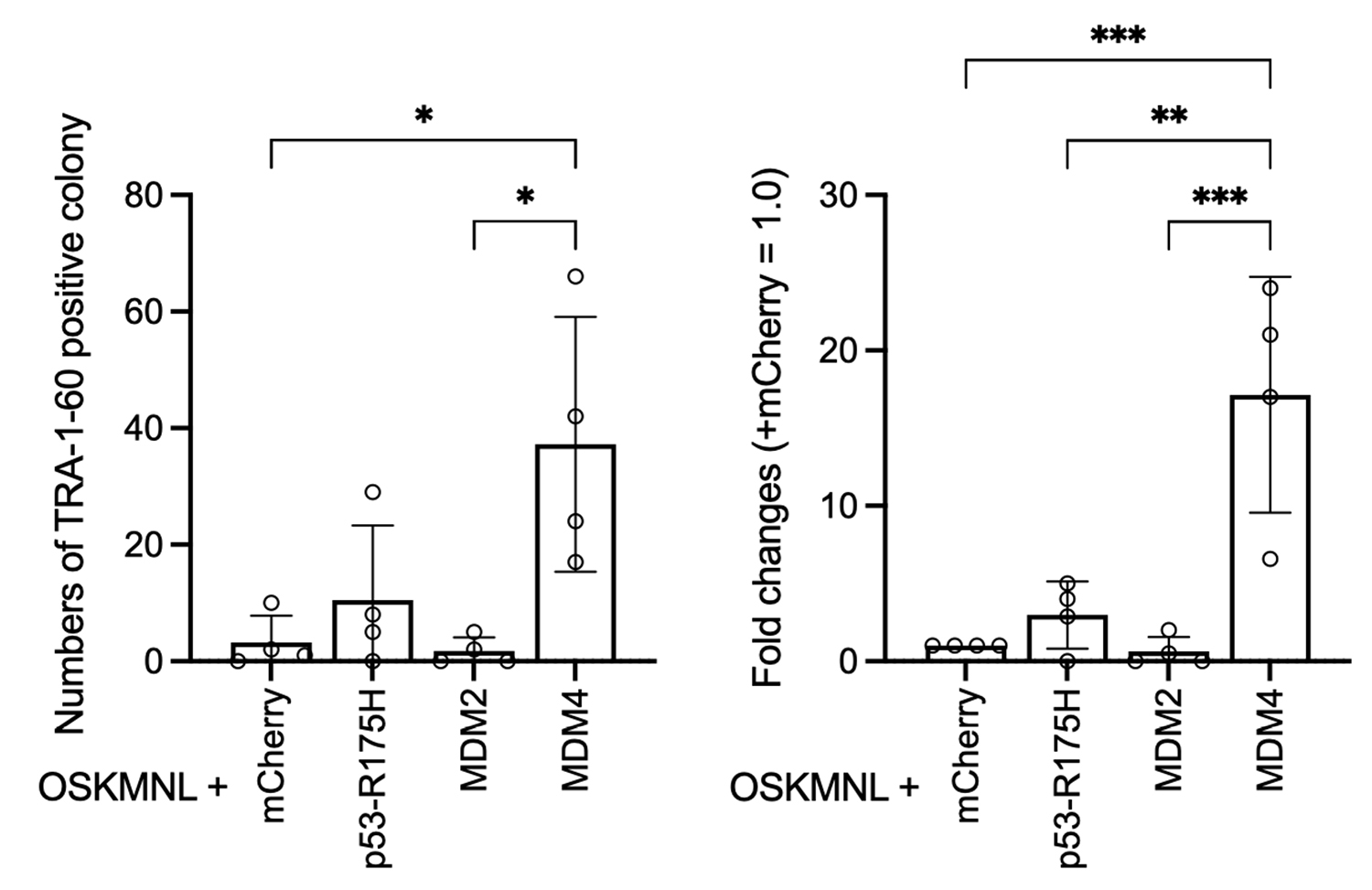
Reprogramming was performed by introducing synthetic mRNAs for reprogramming factors (OSKMNL) along with either mCherry, p53-R175H, MDM2, or MDM4 into PBMCs. The resulting iPSC-like colonies were stained with a TRA-1-60 antibody to quantify the number of colonies (left graph). Based on these values, the fold increase in colony number was calculated relative to the mCherry control set as 1 (right graph). The condition with MDM4 showed a more than 10-fold increase in colony formation.
2. Reproducibility confirmed using PBMCs from multiple donors
The effect of MDM4-S367A was consistently observed in reprogramming experiments using PBMCs from eight healthy donors.
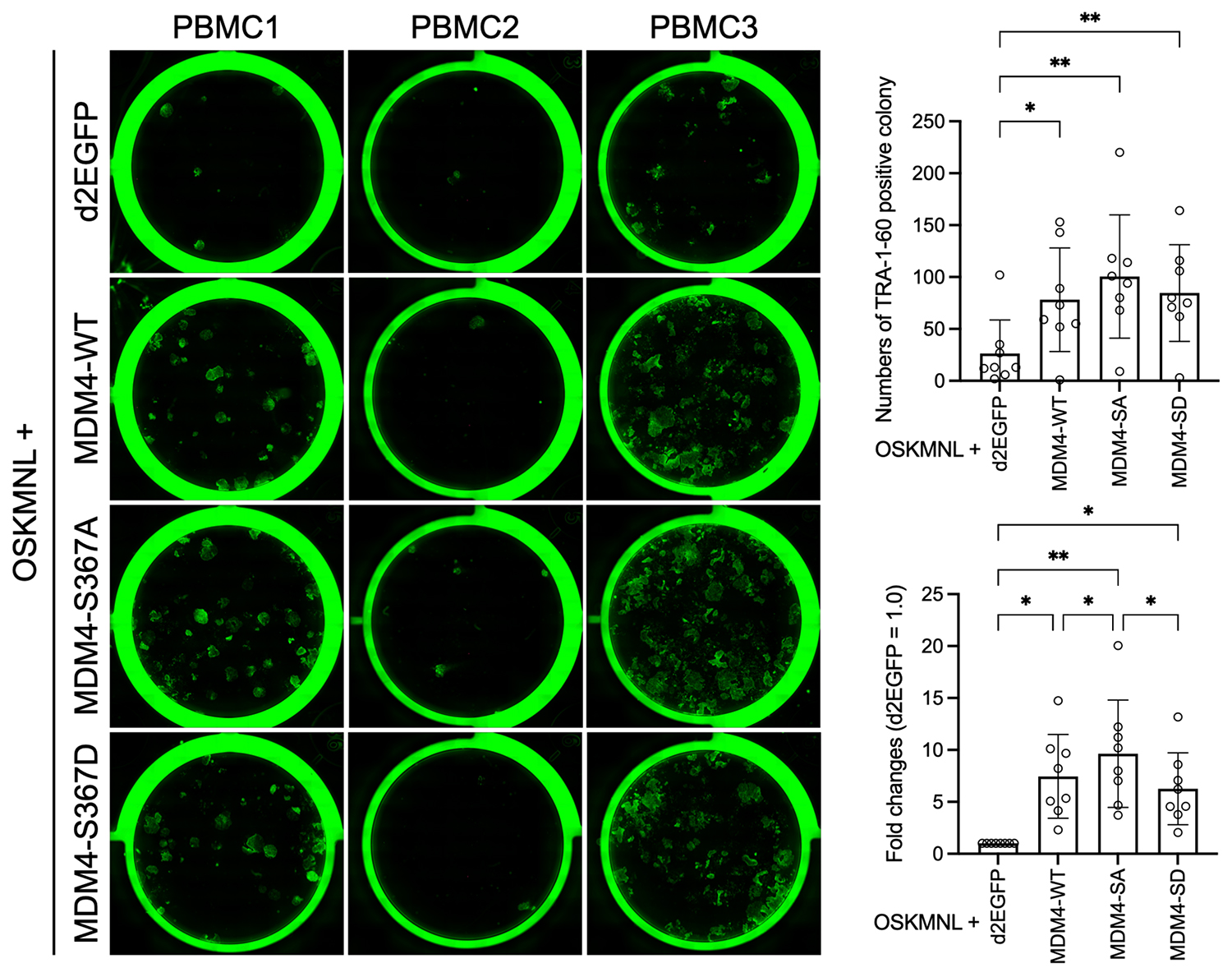
Synthetic mRNAs for d2EGFP, MDM4-WT (wild type), MDM4-S367A (non-phosphorylatable mutant), and MDM4-S367D (phosphomimetic mutant) were introduced into PBMCs along with reprogramming factors (OSKMNL). The resulting iPSC-like colonies were stained with a TRA-1-60 antibody (left panel image; green indicates iPSC-like colonies) to quantify the number of colonies (upper right graph). Based on these counts, fold changes relative to the d2EGFP control group were calculated (lower right graph). The immunostaining results represent three individual donors, while the colony count data were obtained from eight donors.
3. Comprehensive Validation of the Quality of Established iPS Cells
The resulting PBMC-derived iPS cells were confirmed to exhibit key properties of pluripotent stem cells, including the expression of undifferentiated markers, the ability to differentiate into all three germ layers, and a normal karyotype.
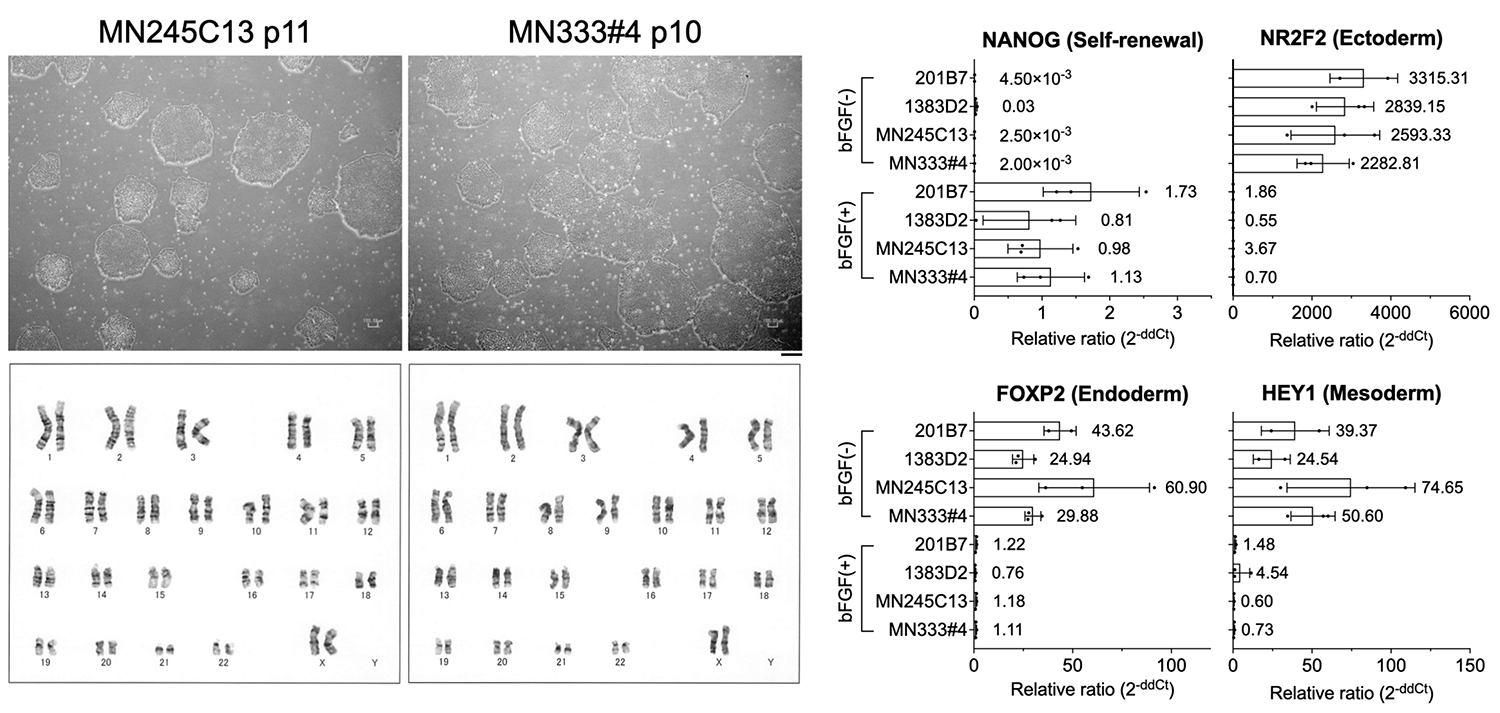
Microscopic images of iPS cells established from PBMCs using synthetic RNA (top left two panels) and their karyotype analysis results (bottom left two panels). Both confirmed a normal karyotype. Gene expression was examined by RT-qPCR under undifferentiated maintenance conditions with bFGF (+) and under spontaneous differentiation conditions without bFGF (−), using the newly established PBMC-iPS cells and previously established, widely used iPS cell lines (201B7 and 1383D2). Expression of markers for all three germ layers was observed under bFGF (−) conditions (right four graphs).
4. Differentiation into corneal epithelial cells demonstrates potential for clinical application
The established PBMC-iPS cells were successfully differentiated into corneal epithelial cells, and expression of markers such as KRT12 and PAX6 was confirmed. These results suggest the potential applicability of these cells in regenerative medicine for vision restoration.
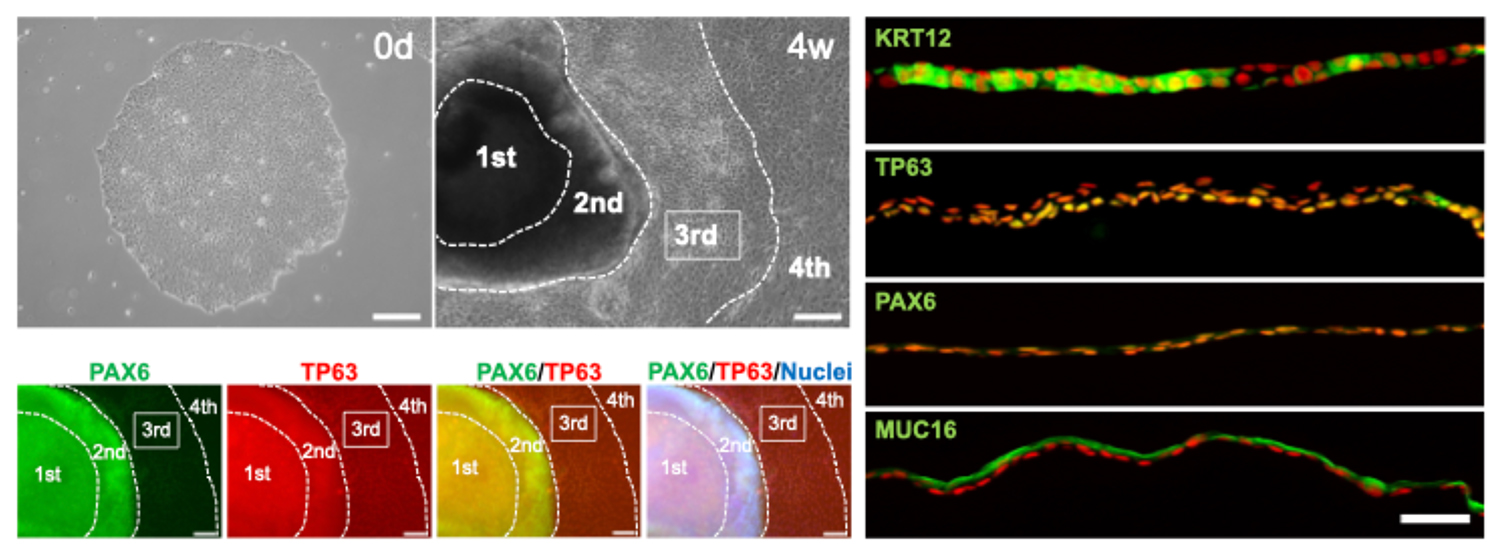
Corneal epithelial tissue was generated using iPS cells established from PBMCs with synthetic RNA. Undifferentiated iPS cells (top-left image, MN328) and differentiated two-dimensional corneal epithelial tissue (SEAM) (top row, second image from the left, MN328) are shown. PAX6-positive and TP63-positive corneal epithelial progenitor cells were successfully induced in the third region (3rd) of the SEAM, as confirmed by immunostaining (bottom-left four images). These progenitor cells were isolated and used to generate corneal epithelial tissue expressing both KRT12 and PAX6, as shown in the right four immunostaining images.
Discussion
- The RNA reprogramming efficiency of PBMCs appears to be severely limited by apoptosis induced through hyperactivated p53 signaling.
- Co-expression of a mutant form of MDM4 (S367A) overcame this reprogramming barrier specific to PBMCs and significantly enhanced the reprogramming efficiency.
- This method demonstrated high reproducibility and practical applicability (validated in other laboratories and facilities).
- Given the clinical compatibility of RNA-based reprogramming and the improved safety profile through MDM4 co-expression, this approach is considered well-suited for future applications in regenerative medicine.
Future Prospects
Moving forward, the researchers aim to apply iPS cells derived from PBMCs to the development of disease models and personalized medicine. Additionally, this protocol is expected to be widely applicable in clinical settings as a safer and more practical method for generating iPS cells.
Paper Details
- Journal: Scientific Reports
- Title: MDM4 enables efficient human iPS cell generation from PBMCs using synthetic RNAs
- Authors: Masato Nakagawa1,2,#,*, Mizuho Nogi1,#, Hatsuki Doi1, Ryuhei Hayashi3,4, Tomohiko Katayama2,3, Hirohisa Ohno1, Megumi Mochizuki1, Karin Hayashi1, Hirohide Saito1,5
*: Corresponding Author
#: Co-First Authors - Author Affiliations:
- Center for iPS Cell Research and Application (CiRA), Kyoto University
- Premium Research Institute for Human Metaverse Medicine (WPI-PRIMe), The University of Osaka
- Department of Ophthalmology, Graduate School of Medicine, The University of Osaka
- Department of Stem Cells and Applied Medicine, Graduate School of Medicine, The University of Osaka
- Institute for Quantitative Biosciences, The University of Tokyo
Dr. Masato Nakagawa's Comment
"Generating iPS cells from PBMCs using only RNA has been a recent challenge. This hurdle has now been removed using MDM4, which regulates the p53 pathway. I believe this protocol will significantly contribute not only to research but also to future clinical applications."
Funding Support
This research was supported by the following funding sources:
-
Japan Agency for Medical Research and Development (AMED)
- Research Center Network for Realization of Regenerative Medicine Core Center for iPS Cell Research
- Acceleration Program of R&D and Implementation for Regenerative Medicine and Cell and Gene Therapy
Core Center for Regenerative Medicine and Cell and Gene Therapy
Core Research Center for Next-Generation Medicine Utilizing Cell and Gene Therapy - iPS Cell Research Fund
-
Japan Science and Technology Agency (JST)
- Fusion Oriented Research for Disruptive Science and Technology (JPMJFR210W)
-
Japan Society for the Promotion of Science (JSPS)
- Grants-in-Aid for Scientific Research (23H03060 and 23K27751)
Intellectual Property Information Related to This Study
The method for producing pluripotent stem cells described in this paper has been granted a patent in Japan.
Glossary
-
iPS Cells (Induced Pluripotent Stem Cells)
Cells generated by introducing specific genes into somatic cells, such as skin or blood cells. They possess the ability to differentiate into various cell types (e.g., neurons, cardiomyocytes, hepatocytes) and are widely applicable in regenerative medicine, disease research, and drug development. -
PBMC (Peripheral Blood Mononuclear Cells)
A type of white blood cell found in the blood, mainly consisting of T cells, B cells, and monocytes, which play crucial roles in immunity. Since they can be obtained through simple blood collection, PBMCs are a convenient source for generating patient-specific iPS cells. -
RNA Transfection (Non-Viral Gene Delivery Method)
A method of temporarily introducing synthetic RNA into cells to express target genes. This virus-free approach is considered highly safe and does not damage the genome. -
Reprogramming (Cellular Reprogramming)
The process of introducing genes into differentiated cells, such as skin or blood cells, to revert them to an undifferentiated, pluripotent state similar to that of a fertilized egg. This technology is crucial for generating iPS cells. -
p53 (Tumor Suppressor Gene)
A crucial protein that induces cell death when abnormalities are detected, thereby preventing cancer development. However, excessive p53 activity during iPS cell generation causes cell death, so its activity must be moderately suppressed. -
MDM4 (Regulator of p53)
A protein that controls p53 activity by suppressing excessive function (a negative regulator). In this study, an RNA encoding a modified form of MDM4 was introduced to safely suppress p53 temporarily and enhance the efficiency of iPS cell generation. -
Pluripotency Markers (Undifferentiated Markers)
Proteins are used as indicators to confirm whether iPS cells have acquired pluripotency (the ability to differentiate into multiple cell types). Markers include TRA-1-60, OCT3/4, and NANOG, which can be detected via immunostaining or PCR. -
Synthetic RNA
Artificially designed and synthesized RNA molecules that temporarily deliver specific genetic information into cells. Due to its rapid degradation and short cellular retention, synthetic RNA is considered a safe gene delivery tool. -
Regenerative Medicine
A therapeutic approach aimed at restoring the function of damaged tissues or organs. iPS cell-derived cells and tissues are expected to be used for supplementing or repairing cells in the body, with potential applications in treating conditions such as neurological disorders, heart disease, and diabetes. -
Three Germ Layers
In early human development, the body is organized into three layers--ectoderm, mesoderm, and endoderm--each of which gives rise to specific tissues, such as the nervous system, muscles, and internal organs. The ability of iPS cells to differentiate into all three germ layers is a key indicator of their pluripotency. -
Karyotype
Refers to the number and structure of chromosomes in a cell's nucleus. In humans, a normal karyotype consists of 46 chromosomes (23 pairs). Maintaining a normal karyotype in iPS cells is critical for ensuring safety and quality control, as abnormalities may increase the risk of tumorigenesis or differentiation defects. -
Corneal Cells
Cells that make up the transparent outermost layer of the eye (the cornea) are essential for maintaining vision. Research on generating corneal cells from iPS cells is at the forefront of regenerative medicine and could lead to treatments for blindness. -
Disease Model
Cells or organisms designed to mimic the characteristics of a specific disease for research purposes. iPS cells derived from patients can be differentiated into disease-relevant cell types (e.g., neurons or cardiomyocytes) to recreate the disease in vitro, facilitating drug discovery and the development of new therapies.






















