CiRA Reporter
CiRA Reporter

Focus
July 7, 2025
Challenges in Treating Incurable Pulmonary Diseases Using iPS Cells

Severe pulmonary diseases, such as interstitial pneumonia (pulmonary fibrosis), often require artificial respiration or lung transplants. According to the Japanese Society of Lung and Heart-Lung Transplantation, 228 patients were registered on Japan’s lung transplant waiting list in 2023, but only 127 received transplants that year. With an average wait time of 2.5 years, many patients face deteriorating health or life-threatening complications. Unfortunately, donor shortages are not unique to Japan—they are a global challenge.
Professor Shimpei Gotoh is using iPS cells to study the pathogenic mechanisms of pulmonary diseases, develop new drugs, and advance regenerative medicine. We spoke with Gotoh about the progress and prospects of his research.

Shimpei Gotoh
What inspired you to become a lung researcher?
After graduating from Kyoto University's Faculty of Medicine, I worked as a medical intern in a hospital, frequently treating patients with pulmonary diseases. Many of these patients had interstitial pneumonia, where the lungs harden and shrink. Seeing patients deteriorate due to limited treatments made me aware of modern medicine's limitations. Their struggles stayed with me, driving my research into pulmonary disease treatment.
That motivated me to enroll in Kyoto University's Graduate School of Medicine, where I focused on basic research into pulmonary diseases. Even during my undergraduate years, I was fascinated by basic research, and I hoped that studying from the ground up could help me discover new treatments.
What was the situation like when you began your research?
When I entered graduate school, research on lung regeneration using iPS cells was still in its early stages—only about three years had passed since Professor Shinya Yamanaka's groundbreaking discovery. At that time, generating lung cells from iPS cells for regenerative medicine research remained an enormous challenge. With CiRA's support, I learned iPS cell culturing and differentiation techniques. After consulting many CiRA researchers, we successfully produced lung progenitor cells.
We subsequently created lung organoids (mini lungs) from these progenitor cells and were among the first to publish such findings. I am grateful for CiRA's support in making this possible.
What kind of research are you conducting with lung organoids?
Human lungs contain about 300 million alveoli, structures in the lungs responsible for exchanging oxygen and carbon dioxide. They consist of two main types of epithelial cells: Type 1 and Type 2 alveolar epithelial cells. Type 2 alveolar epithelial cells differentiate into Type 1 alveolar epithelial cells, which are flattened, and secrete lung surfactant—a substance that helps maintain lung expansion.
We successfully developed a method to efficiently generate alveolar organoids (Figure 1) from iPS cells that replicate all of these structures and functions. Using iPS cells from interstitial pneumonia patients, we created alveolar organoids and studied the disease at a cellular level. We also induced interstitial pneumonia in these organoids by administering disease-causing drugs, effectively recreating the condition in a Petri dish. Using this model, we are screening for potential therapeutic drugs.
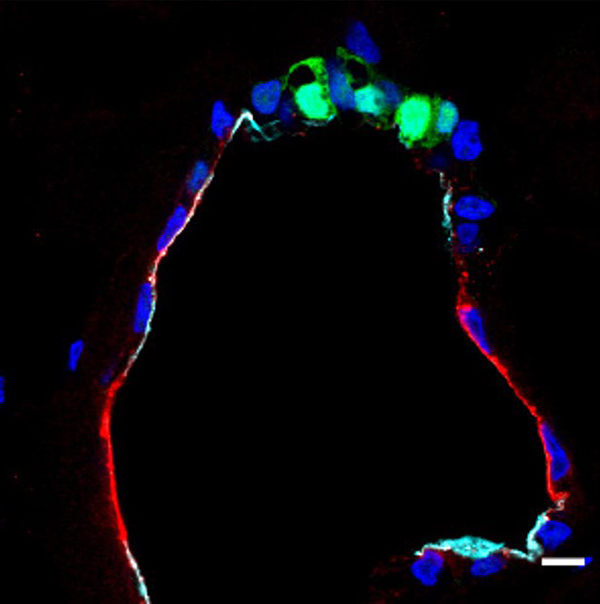
Figure 1: Cross-section of an iPS-derived alveolar organoid.
Green shows Type II alveolar cells (round-shaped) and red shows Type I alveolar cells (flattened). The black region inside the organoid is filled with secreted substances such as pulmonary surfactant.
You are also researching the SARS-CoV-2 virus. Can you explain more?
We developed a technique to culture alveolar and airway organoids (Figure 2) in "2.5-dimension" (2.5D) with specially engineered plates (Figure 3). Typically, organoids are grown in a 3D gel matrix, thus making viral infection difficult. However, our 2.5D culture system partially exposes the organoid's surface, facilitating more efficient viral infections.
Using this technology, we collaborated with former CiRA Junior Associate Professor Kazuo Takayama to study how the SARS-CoV-2 virus infects lung cells. We discovered that the virus primarily infects Type 2 alveolar epithelial cells but also infects airway organoids extensively. Different variants of the virus infect cells differently. For example, the highly infectious Delta variant strongly infected both alveolar and airway organoids, while the Omicron variant had significantly reduced infectivity. We can also use this technology to study other viruses in the future.
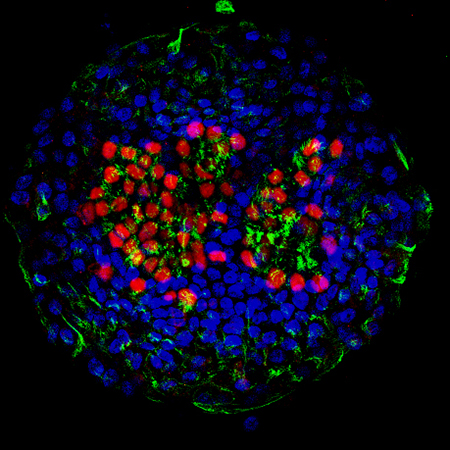
Figure 2: Airway organoid derived from iPS cells.
The red area in the center indicates the nuclei of ciliated cells, while the surrounding green area indicates cilia.
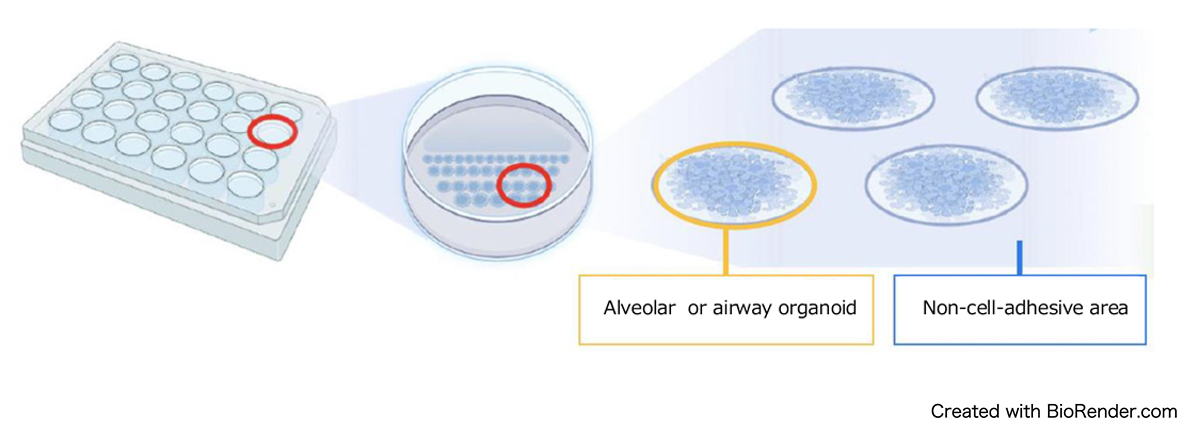
Figure 3: An illustration of alveolar and airway organoids culture in 2.5D.
Are you studying other pulmonary diseases?
Yes, we are also researching diseases that affect the cilia—tiny hair-like structures in the airways that help clear out foreign particles, bacteria, and viruses.
One such condition is Primary Ciliary Dyskinesia (PCD), a rare genetic disorder that causes chronic cough, persistent phlegm, and repeated respiratory infections such as pneumonia. Diagnosing PCD is challenging, and no effective treatments currently exist. Furthermore, taking repeated cell samples from patients to study the disease is a major burden on them.
To address this, we created iPS cells from patient blood samples to generate ciliated cells in the airway, effectively replicating the disease in the laboratory. This breakthrough has allowed us to identify the genetic causes of PCD and begin searching for new drug candidates.
A key achievement was creating airway organoids with ciliated cells from iPS cells. However, traditional culturing methods have limitations: when grown in Petri dishes, the cilia fail to align in a coordinated direction. In the human body, cilia must move in unison to efficiently clear out debris and mucus.
To solve this problem, we collaborated with researchers from the Graduate School of Engineering at Kyoto University to develop a microfluidic "airway chip" replicating airway functions. This chip allows us to culture airway epithelial cells derived from iPS cells under conditions with fluidic flow. When grown on this chip, the ciliated cells naturally align and coordinate their movements, even after the fluid flow stops. This innovation vastly improves our ability to study ciliary function and develop treatments for PCD.
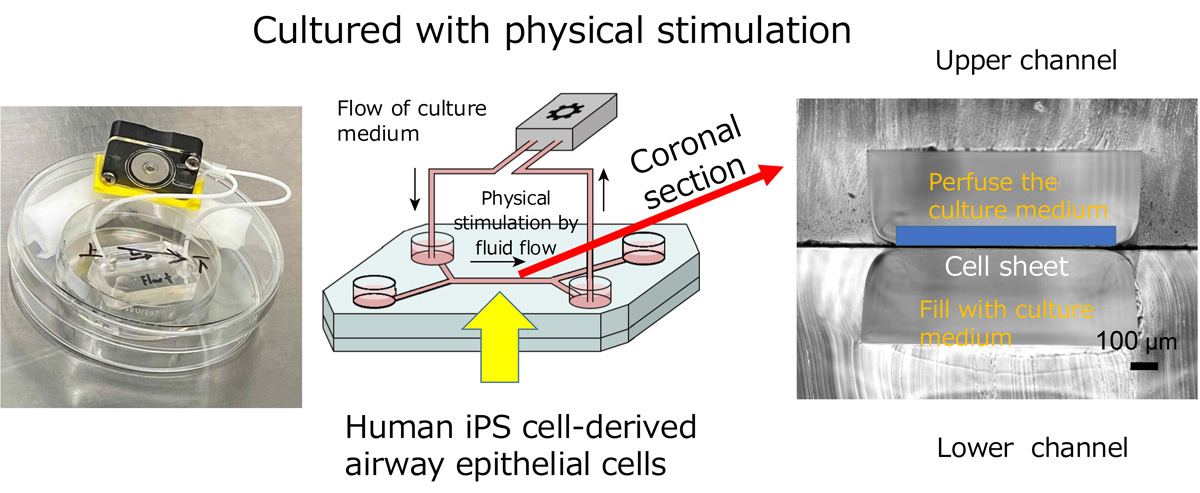
Figure 4: An example of the "airway chip" and an illustration explaining how it works.
What are your goals for the future?
Our main objectives are developing new drugs for pulmonary diseases and regenerating lung tissue using iPS cells. First, our immediate goal is to identify effective drugs to help treat various pulmonary conditions. Looking further ahead, we hope to use iPS-derived cells to regenerate damaged lung tissues.
Currently, we are studying lung regeneration by transplanting human iPS-derived progenitor cells into mice and assessing their integration and function. If this technique can be applied to humans, it could provide a groundbreaking alternative to lung transplantation.
We also aim to advance personalized medicine. Patients with the same disease often experience varying conditions. By identifying drugs that work best for specific patients, we can tailor treatments to their needs.
One of the biggest strengths of iPS cells is that we can use patient-derived cells and research using cells with the patient's genetic background, which makes it easier to develop personalized treatments. For rare diseases, discovering effective treatments for individual patients could reduce the risks they face while waiting for lung transplants.
My goal has always been to treat incurable pulmonary diseases, with bringing iPS-derived lung cells into clinical use as the next step. Ultimately, I hope to create a fully functional lung using iPS cells—a vision I work toward every day.
-
Profile: Professor Shimpei Gotoh
Shimpei Gotoh was born in Kyoto Prefecture. After graduating from Kyoto University's Faculty of Medicine, he completed his residency and clinical fellowship in the Department of Respiratory Medicine and earn his Ph.D. at the Kyoto University Graduate School of Medicine. He then worked as an Assistant Professor at the Kyoto University Hospital and an Associate Professor at Kyoto University's Graduate School of Medicine before being appointed Professor at CiRA in 2022. As a hobby, he enjoys taking on new challenges. -
Interviewed and written by Yoko Miyake
Science Communicator, CiRA International Public Communications Office
(Translation: Kelvin Hui Ph.D., CiRA Research Promoting Office)






















