
Research Activities
Research Activities
Publications
May 03, 2017
The metabolism of cell reprogramming
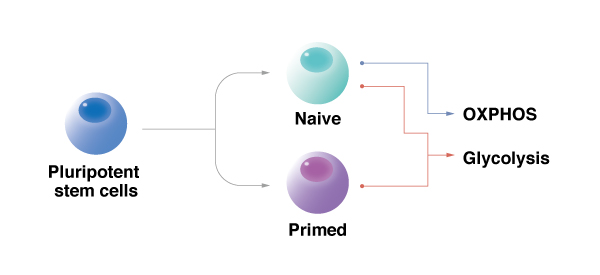
In reprogramming, the cell metabolism determines the quality of the pluripotency.
Cells that only exercise glycolysis have prime pluripotency. Those that exercise glycolysis and OXPHOS have naive pluripotency.
regulate cell metabolism for pluripotency
During cell reprogramming, a number of molecular and cellular events must occur for the cell to become pluripotent. One example is change in the metabolism, since the pluripotent state brings new energy requirements for the cell. In their most recent work, Junior Associate Professor Takuya Yamamoto and his lab report two transcription factors, Zic3 and Esrrb, have critical roles in regulating the metabolism during cell programming, providing new details about how to convert a cell to the pluripotent state.
Yamamoto explains that metabolism is an essential part of cell identity and cell reprogramming. "Glycolysis and OXPHOS are partly antagonistic metabolic pathways and their balance should be strictly regulated during reprogramming."
The study shows how Zic3 and Esrrb work together for this regulation. Zic3 activates glycolysis and inhibits OXPHOS. Esrrb, on the other hand, is necessary to activate OXPHOS and at the same time augments the glycolysis activation done by Zic3. This synergy, the study shows, is because Zic3 recruits Esrrb to common binding sites on a gene and may explain why other factors besides Esrrb that activate OXPHOS but do not enhance glyolysis are far less effective at reprogramming.
The findings suggest new molecular networks for metabolic reprogramming. While other proteins have been found capable of forcing the glycolytic metabolism cells, the Zic3-Esrrb approach for a different type of pluripotency.
"HIF proteins facilitate reprogramming cells into the primed state by activating glycolysis," said Dr. Masamitsu Sone, who conducted the experiments, "but the Zic3-Esrrb synergy works to obtain naive state independently of HIF."
Naive and primed pluripotency is thought to describe the state of an embryo before and after implantation onto the uterine wall (i.e. before and after pregnancy), respectively. Scientists have had relative ease acquiring both states in mouse cells, but the naive state has proven more elusive in human cells. Furthermore, both glycolysis and OXPHOS are activated in naive cells, whereas only glycolysis is activated in primed cells. Consistently, Esrrb is known to bind to factors that promote naive pluripotency during reprogramming, while Zic3 binds to factors associated with primed pluripotency.
Overall, the study suggests a rigid order of metabolic events that must occur to capture the naive state, with glycolysis happening early to initiate the process and OXPHOS happening later while glycolysis is retained to complete it.
"The transition from naive to primed pluripotency is fundamental to developmental biology," noted Yamamoto. "Our findings show OXPHOS is an important event to establish naive pluripotency and could provide new molecular pathways for this transition."
Paper Details
- Journal: Cell Metabolism
- Title: Hybrid cellular metabolism coordinated by Zic3 and Esrrb synergistically enhances induction of naive pluripotency
- Authors: Sone M1,2, Morone N2,3, Nakamura T1, Tanaka A1, Okita K1, Woltjen K1,4, Nakagawa M1, Heuser JE2, Yamada Y1,2, Yamanaka S1,5 and Yamamoto T1,2,6
- Author Affiliations:
- Center for iPS Cell Research and Application (CiRA), Kyoto University, Kyoto, Japan
- Institute for Integrated Cell-Material Sciences (iCeMS), Kyoto University, Kyoto, Japan
- MRC Toxicology Unit, University of Leicester, Leicester, UK
- Hakubi Center for Advanced Research, Kyoto University, Kyoto, Japan
- Gladstone Institute of Cardiovascular Disease, San Francisco, CA, USA
- AMED-CREST, AMED, Tokyo, Japan






















