
Research Activities
Research Activities
Publications
April 13, 2018
One gene changes cell identity
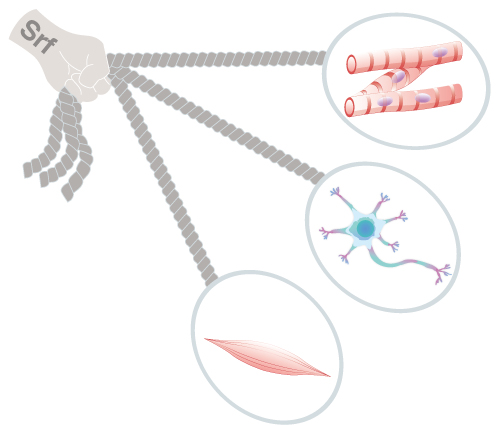
The expression of Srf1 disrupts the identity of various types of cells.
The expression of Srf1 disrupts the identity
of various types of cells.
Cells are defined by their function and shape. Skin cells are different from stomach cells which are different from spleen cells which are different from skeletal cells. Accordingly, each cell type expresses its own set of cell-identity genes, genes that CiRA Junior Associate Professor Keisuke Okita calls "roadblocks."
"The maintenance of cell identity is crucial for health, and its loss is associated with aging and cancer. Roadblocks are factors that maintain cell identity and block reprogramming," he said.
Okita and his CiRA colleague, Dr. Takashi Ikeda, both experts in reprogramming, considered if there are any universal factors that suppress roadblocks regardless of the cell type. In the new study, they and colleagues show that serum response factor (Srf) compromises the identity of several different types of mouse cells, including neural progenitor cells, hepatoblasts and uretic bud cells.
Srf responds to mechanical signals in the cell, like changes in cell shape, to activate the expression of other genes. As a result, it has a very important role in embryo development, as the embryo undergoes constant physical change to grow into a fully formed body, and also in adult cells like muscle.
"In reprogramming, roadblocks are downregulated. We found that downregulating the β-actin gene, Actb, contributed to reprogramming regardless of the cell," said Ikeda.
β-actin is a fundamental molecule for cell morphology. Ikeda found that changes in Actb expression led to changes in Srf activation. Srf consequently bound to roadblock genes through its MADS-box domain, suppressing their expression and thus compromising cell identity. This compromise then made the cell susceptible to being reprogrammed to the pluripotent state, i.e. iPS cells.
The study further shows the nuclear effects of Srf binding to a gene. The binding decreased the levels of H3K27ac, a chromatin modifier that enhances gene transcription, on roadblock genes. The genes were physically displaced from the interior of the nucleus to the periphery. Interestingly, in terms of reprogramming, this effect was exclusive to roadblock genes, as Srf did not show a propensity to enhance the expression of genes responsible for pluripotency.
The scientists also found that Srf activation could have pathological repercussions. Overexpressing Srf in mice led to inflammatory conditions such as colitis and also evidence of metaplasia and dysplasia, which are symptoms associated with cancer. This is not a great surprise, since roadblock genes keep the cell state constant, and reprogramming involves the activation of oncogenic genes in order to convert the cell into one that is pluripotent.
Based on the all the above, Ikeda explained that Srf could be a common factor in diseases associated with cells behaving unstably.
"During early development, cells undergo many changes. But in the adult body these changes must stop so that each cell type carries its function. In many diseases, cell function is compromised. Srf misactivation could be a cause," he said.
Paper Details
- Journal: Nature Communications
- Title: Srf destabilizes cellular identity by suppressing cell-type-specific gene expression programs
- Authors: Takashi Ikeda1, Takafusa Hikichi1, Hisashi Miura2, Hirofumi Shibata1, Kanae Mitsunaga1, Yosuke Yamada1,3, Knut Woltjen1,4, Kei Miyamoto5, Ichiro Hiratani2, Yasuhiro Yamada1,6,7, Akitsu Hotta1,7, Takuya Yamamoto1,7,8, Keisuke Okita1, and Shinji Masui1,9
- Author Affiliations:
- Center for iPS Cell Research and Application (CiRA), Kyoto University, Kyoto, Japan
- RIKEN Center for Developmental Biology (CDB), Kobe, Japan
- Department of Diagnostic Pathology, Kyoto University Hospital, Kyoto, Japan
- Hakubi Center for Advanced Research, Kyoto University, Kyoto, Japan
- Laboratory of Molecular Developmental Biology, Faculty of Biology-Oriented Science and Technoloyg, Kindai University, Wakayama, Japan
- Division of Stem Cell Pathology, Center for Experimental Medicine and Systems Biology, Institute of Medical Science, University of Tokyo, Tokyo Japan
- Institute for Integrated Cell-Material Sciences (WPI-iCeMS), Kyoto University, Kyoto, Japan
- AMED-CREST, AMED, Tokyo, Japan
- Core Research for Evolutional Science and Technology (CREST), Japan Science and Technology Agency (JST), Saitama, Japan






















