
Research Activities
Research Activities
Publications
October 11, 2023
Identification of potential putative drug compounds for treating interstitial lung disease
Interstitial lung disease (ILD) is a group of diseases caused by chronic inflammation and fibrosis of the lungs. Such chronic lung damage is irreversible and may cause severe respiratory failure. A major challenge to overcoming ILD is the wide range of causal factors, including prolonged environmental exposure to hazardous materials, such as asbestos, or primary diseases, such as certain autoimmune disorders. Furthermore, genetic studies have also identified some mutations associated with alveolar epithelial type 2 (AT2) cells that can cause progressive pulmonary fibrosis.
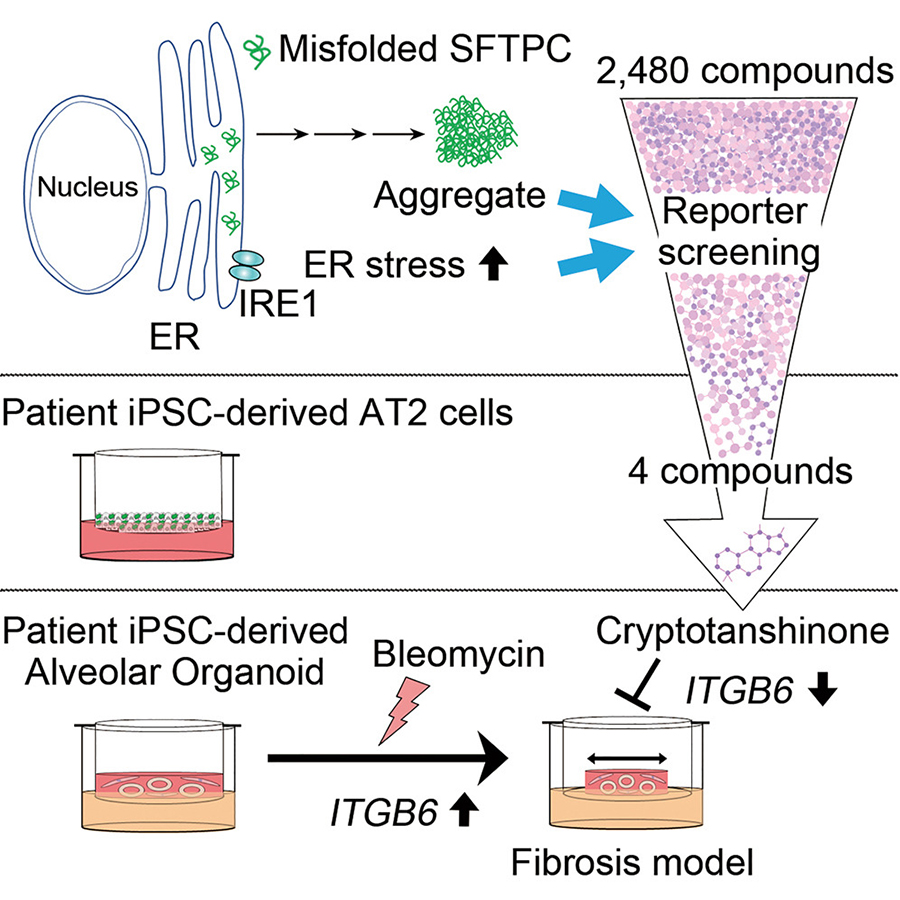
Specifically, mutations in genes involved in surfactant metabolism and functions, including surfactant protein C (SFTPC ), surfactant protein A1/A2 (SFTPA1/A2 ), and ATP binding cassette subfamily A member 3 (ABCA3 ) have been linked to ILD. Critically, many mutations in the SFPTC gene cause protein misfolding, thus leading to protein accumulation and aggregation, aggresome formation, and endoplasmic reticulum (ER) stress. In the recent study, the collaborative research team exploited two molecular ILD phenotypes--ER stress and SFTPC aggregation--to establish a small molecule screen to identify potential new drugs for ILD treatment and validated the efficacy of these newly identified compounds using ILD patient-specific induced pluripotent stem cell (iPSC)-derived lung organoids with an ILD-associated SFTPC mutation. As a result of their research efforts, Cryptotanshinone (CPT) was identified as a putative therapeutic agent for ILD.
The researchers first designed a primary small molecule screen using a reporter system based on XBP1 splicing, a classical indicator of ER stress, in HEK293 (a human embryonic kidney cell line) expressing wild-type or ILD-associated L188Q mutant SFTPC. After confirming that the SFTPC mutation induces various ER stress markers, the team screened nearly 2,500 compounds from the Kyoto University chemical library and identified 65 candidate compounds that could reverse the ER stress induced by the mutant SFTPC protein. The team subsequently focused on these candidates by testing their ability to reduce ER stress and SFTPC protein aggregation caused by another mutation (Y104H) and ultimately selected four compounds (#562, #775, #2035, and #2352) for detailed analysis using iPSC-derived AT2 cells.
The researchers generated iPSC lines from the blood sample of an ILD patient with a heterozygous SFTPC Y104H mutation and a "rescued" iPSC line through CRISPR/Cas9-mediated genome editing (without the SFTPC Y104H mutation) and differentiated them into AT2 cells in an air-liquid interface. They found that compound #2035 normalized SFTPC Y104H mutant protein accumulation to wild-type levels in the iPSC-derived AT2 cells. The research team further studied compound #2035, revealed as CPT, for its beneficial effects in HEK293 and A549 (a lung epithelial cell line) cells and discovered that it effectively reduced protein aggregates and cell death caused by the SFTPC Y104H mutant.
Finally, the researchers validated the efficacy of CPT using an in vitro pulmonary fibrosis model using fibroblast-dependent alveolar organoids comprised of patient-specific iPSC-derived alveolar epithelial cells and human fetal lung fibroblasts. They first identified that the alveolar epithelial cells of diseased (SFTPC Y104H) alveolar organoids exhibited a gene expression profile consistent with ILD, specifically inflammation-, lung fibrosis-, and protein folding-related gene sets' upregulation. Notably, no significant gene expression profile changes were observed in the fibroblasts of these alveolar organoids, indicating changes to the alveolar epithelial cells were insufficient to elicit a response by the fibroblasts. Crucially, the researchers observed CPT treatment of the mutant alveolar organoids to reduce ITGB6 expression, a gene encoding an epithelial-specific receptor upregulated typically in response to epithelial injury, thus suggesting that this newly identified compound has beneficial effects on ILD-associated mutant AECs. Separately, CPT demonstrated significant protective effects on alveolar organoids subjected to bleomycin treatment, a chemotherapeutic drug known to cause pulmonary damage, used to enhance the injury to alveolar epithelial cells.
Altogether, by using patient-specific iPSCs, the research team more accurately modeled ILD pathology and validated the efficacy of CPT, identified by high-throughput screening, for treating ILD. Although further work is required, CPT represents a candidate ILD treatment strategy, as the limited therapeutic options currently available principally focus on reducing fibrosis. CPT, in contrast, acts directly on alveolar epithelial cells, which are involved in the early stages of ILD and may thus be even better at preventing ILD progression.
Paper Details
- Journal: iScience
- Title: Cryptotanshinone is a candidate therapeutic agent for interstitial lung disease associated with a BRICHOS-domain mutation of SFTPC
- Authors: Motoyasu Hosokawa1,2,9, Ryuta Mikawa3,4,5,9, Atsuko Hagiwara1,3, Yukiko Okuno6, Tomonari Awaya1, Yuki Yamamoto3,4, Senye Takahashi4,5, Haruka Yamaki3,5, Mitsujiro Osawa5, Yasuhiro Setoguchi7, Megumu K. Saito5, Shinji Abe8, Toyohiro Hirai3, Shimpei Gotoh3,4,5*, and Masatoshi Hagiwara1*
*:Corresponding authors - Author Affiliations:
- Department of Anatomy and Developmental Biology, Graduate School of Medicine, Kyoto University
- Department of Developmental Biology and Functional Genomics, Ehime University Graduate School of Medicine
- Department of Respiratory Medicine, Graduate School of Medicine, Kyoto University
- Department of Drug Discovery for Lung Diseases, Graduate School of Medicine, Kyoto University
- Department of Clinical Application, Center for iPS Cell Research and Application (CiRA), Kyoto University
- Medical Research Support Center, Graduate School of Medicine, Kyoto University
- Department of Respiratory Medicine, Graduate School of Medical and Dental Sciences, Tokyo Medical and Dental University (TMDU)
- Department of Respiratory Medicine Tokyo, Medical University Hospital
- These authors contributed equally






















