
Research Activities
Research Activities
Publications
March 13, 2024
A novel strategy to efficiently differentiate into subtype-specific cardiomyocytes from human iPS cells
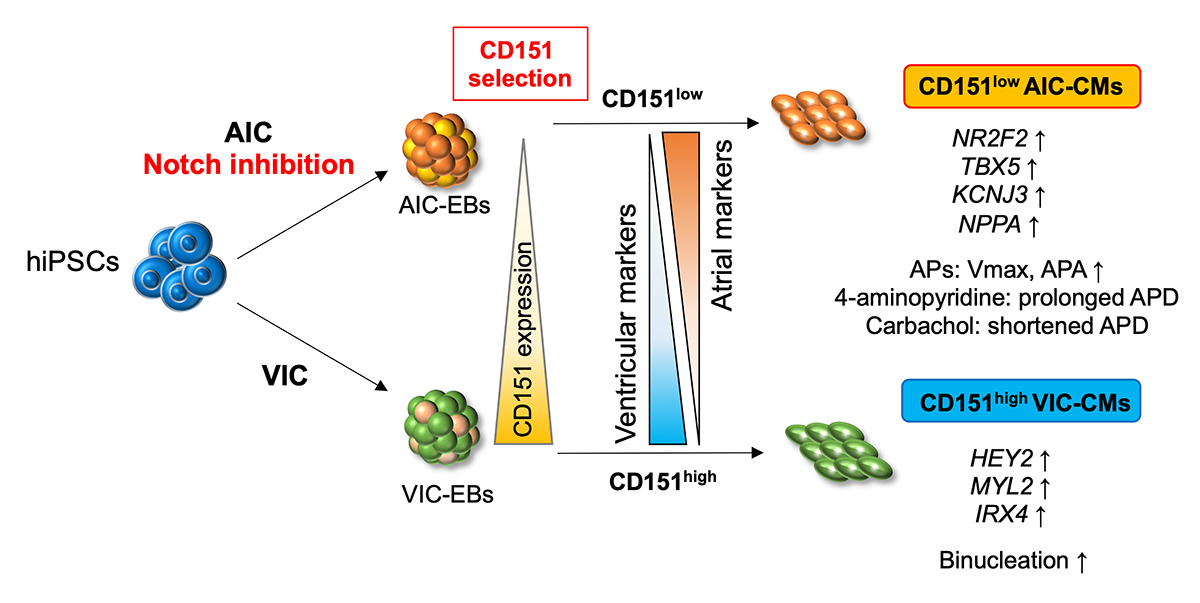
Cardiovascular disease is one of the most prevalent causes of death worldwide. hiPSC-derived cardiomyocytes are promising resources for drug discovery and regenerative medicine. Our heart is composed of several subtypes of cardiomyocytes. Specifically, the ventricle and atrium consist of ventricular cardiomyocytes (VCMs) and atrial cardiomyocytes (ACMs), respectively. Conventional protocols for producing cardiomyocytes result in the generation of heterogeneous populations mixed with these subtypes, so new methods to obtain each subtype with high efficiency were required for clinical application. The research team, therefore, sought to identify subtype-specific cell surface markers to isolate ACMs and VCMs.
First, the researchers confirmed although conventional differentiation methods predominantly generated the targeted subtypes, hiPSC-derived subtype-specific cardiomyocyte cultures still contain significant contamination by undesired cells (i.e., ACMs in VCM culture and VCMs in ACM culture).
Next, the researchers screened over 200 cell surface proteins with those ACM- or VCM-dominant cell populations to identify differentially expressed markers to potentially use for subtype purification. Through this approach, they found CD151 to distinguish between these two cardiomyocyte subtypes. The researchers further analyzed cells with high and low expression levels of CD151 to discover that atrial-related genes were highly expressed in the CD151low population and ventricular genes were expressed at high levels in the CD151high population. Furthermore, the research team examined the electrophysiological properties of CD151high and CD151low subpopulations to determine whether they are functional ACMs or VCMs. Remarkably, they detected atrial-type action potentials only in ACM-differentiated CD151low cells. On the other hand, the cells showing ventricular-type action potentials were enriched in the CD151high population. These results show that ACMs and VCMs can be distinguished in a heterogeneous hiPSC-derived cardiomyocyte population by CD151 expression levels.
Cardiomyocyte proliferation stops after birth, even as their nuclei continue to divide during development, thus leading to binucleation (more than one nucleus per cell). Global gene expression analysis of CD151high VCMs generated from the VCM-specific differentiation protocol showed an enrichment of genes related to cell cycle and mitosis in these VCMs. Flow cytometric analysis further showed while cell proliferation was not different between the CD151high and CD151low subpopulations, an increase of binucleated cells was detected in VCMs with high levels of CD151. Additional experiments also revealed the upregulation of several ventricular genes expressed in adult hearts in CD151high VCMs. Thus, in combination with the observed increase in binucleation, CD151high VCMs exhibit many characteristics suggestive of advanced differentiation.
The research team also examined the global expression patterns of CD151high and CD151low ACM and VCM subpopulations to elucidate the molecular mechanisms underlying this CD151-based selectivity. They identified through this analysis a prominent molecular signature for the Notch signaling pathway and discovered a correlation between the gene expression of NOTCH -related molecules and CD151 levels. While pharmacologic disruption of Notch signaling using the gamma-secretase inhibitor LY411575 did not influence CD151 levels, it promoted atrial differentiation. The research team also demonstrated that LY411575 treatment of ACMs induced by the atrial differentiation protocol enhanced the subpopulation of cells producing atrial-like action potentials, thus suggesting the combination of CD151-based selection and Notch inhibition significantly improves the purification of functional ACMs.
Although the precise relationship between CD151 expression and cardiomyocyte differentiation remains unknown, this study demonstrated its utility in selecting cell subpopulations to attain more homogeneous and functionally mature ACMs and VCMs. In addition, the researchers revealed the crucial role of Notch signaling in ACM differentiation and maturation. Altogether, these findings offer novel mechanistic insights into cardiomyocyte differentiation and contribute to improvements to current protocols for generating specific cardiomyocyte subtypes from hiPSCs for basic research and biomedical applications.
Paper Details
- Journal: Communications Biology
- Title: CD151 expression marks atrial- and ventricular- differentiation from human induced pluripotent stem cells
- Authors:
Misato Nakanishi-Koakutsu1-4, Kenji Miki1,5,6,*, Yuki Naka1,2, Masako Sasaki1,2, Takayuki Wakimizu1,2, Stephanie C Napier2,7, Chikako Okubo1, Megumi Narita1, Misato Nishikawa1, Reo Hata1, Kazuhisa Chonabayashi1,9, Akitsu Hotta1,2, Kenichi Imahashi2,7, Tomoyuki Nishimoto2,8, Yoshinori Yoshida1,2,*
*: Corresponding Authors - Author Affiliations:
- Center for iPS Cell Research and Application (CiRA), Kyoto University
- Takeda-CiRA Joint program (T-CiRA)
- Division of Cardiology, Johns Hopkins University School of Medicine
- Department of Surgery, Johns Hopkins School of Medicine
- Center for Organ Engineering, Department of Surgery, Massachusetts General Hospital
- Department of Surgery, Harvard Medical School
- Global Advanced Platform, Research, Takeda Pharmaceutical Company Limited
- Orizuru Therapeutics Incorporated
- Department of Hematology and Oncology, Graduate School of Medicine, Kyoto University






















