Research Activities
Research Activities
Principal Investigators
Dept. of Life Science Frontiers
Mitinori Saitou (Professor)

Mitinori Saitou M.D., Ph.D.
Research Overview
Germ cells differentiate into sperm and egg cells (oocytes), the fusion of which creates a new individual. Understanding the mechanisms involved in the development of germ cells will provide a direct key to understanding the mechanisms of genetic information transmission and epigenetic control and thereby cast light on the mechanisms underlying infertility, hereditary disease, and epigenome abnormalities.
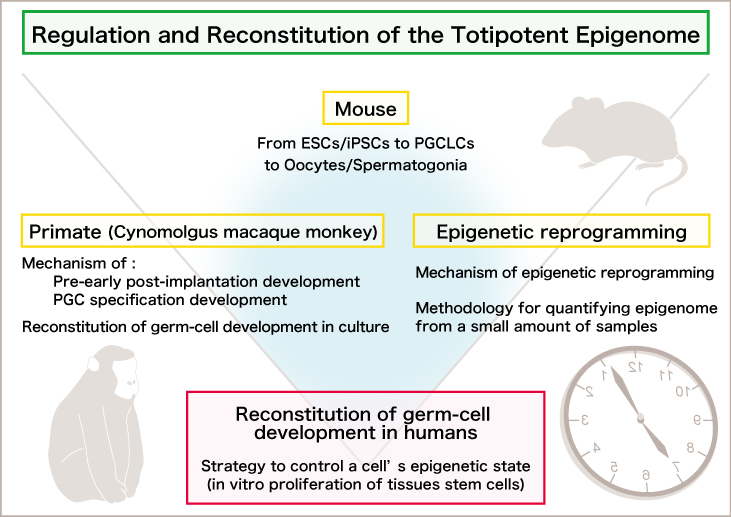
We have been investigating the mechanism for germ cell development and have shown that mouse primordial germ cell-like cells (mPGCLCs) induced from embryonic stem cells (mESCs)/induced pluripotent stem cells (miPSCs) have a robust capacity both for spermatogenesis and oogenesis and for contributing to offspring. These works form the basis for elucidating key mechanisms during germ cell development, such as epigenetic reprogramming, sex determination, meiotic entry, and nucleome programming.
By investigating the development of cynomolgus monkeys as a primate model, we have defined a developmental coordinate of pluripotency among mice, monkeys, and humans, identified the origin of the primate germ cell lineage in the amnion, and have elucidated the X-chromosome dosage compensation program in primates.
Recently, based on these achievements, we have developed a technology to induce human iPS cells into human primordial germ cell-like cells (hPGCLCs), clarified the formation mechanism of human germ cells, and revealed that the formation mechanisms of human and mouse germ cells differ in many evolutionary aspects. Furthermore, we have succeeded in differentiating monkey and human oogonia into primordial follicles ex vivo, and in inducing monkey ES cells into monkey primordial germ cell-like cells, which were then differentiated into fetal oocytes at prophase I. Moreover, recently, using cytokines and other factors, we successfully induced epigenetic reprogramming in human primordial germ cell-like cells, differentiating them into pre-spermatogonia and oogonia. During this process, cells derived from human primordial germ cell-like cells maintain a stable karyotype and are amplified more than 10^10-fold. These achievements promote the elucidation of human germ cell development mechanisms and form a foundation for further developing human in vitro gametogenesis research.
We aim to extend the study of human germ cell development based on these models.